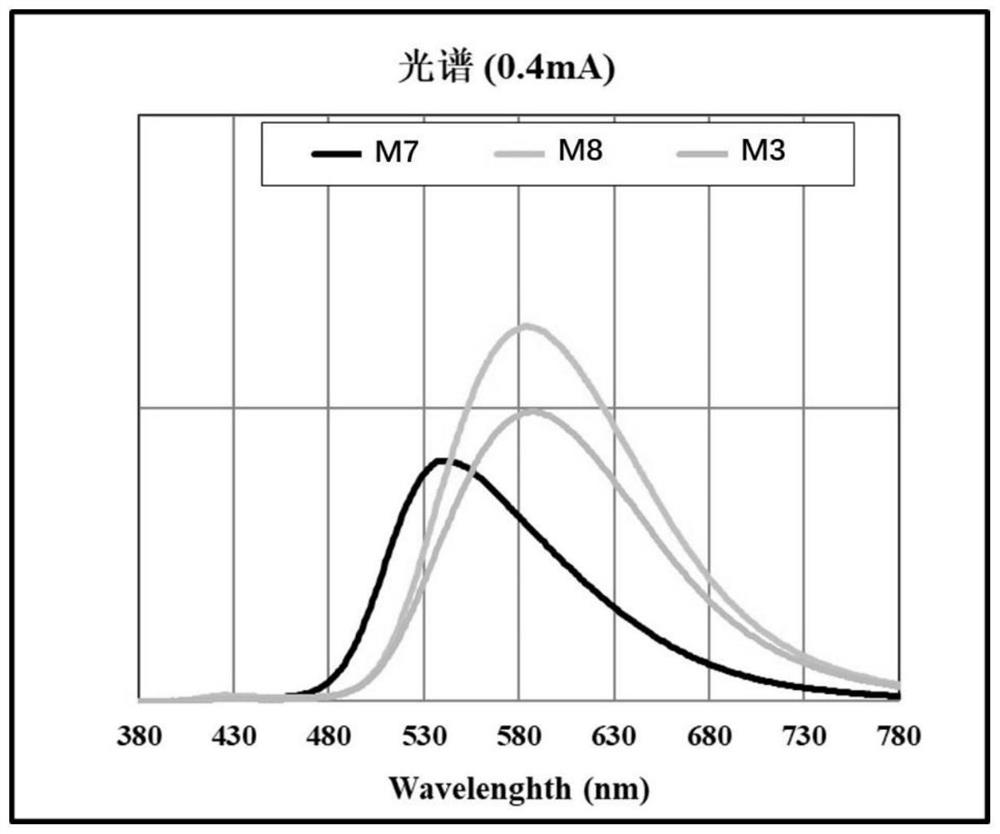
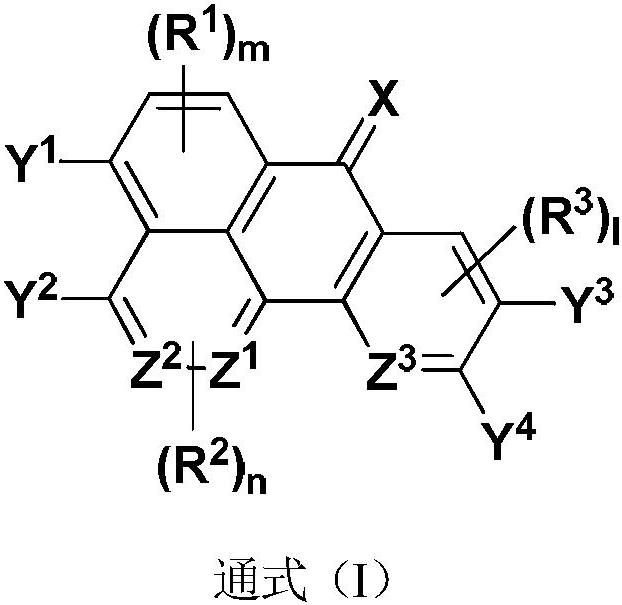
Thermally-induced delayed fluorescence semiconductor, and preparation method and application thereof
A thermally-induced delayed fluorescence, semiconductor technology, applied in semiconductor/solid-state device manufacturing, semiconductor devices, chemical instruments and methods, etc., can solve the problems of poor color purity, wide EL spectrum, missing spectral fine structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The present invention also discloses a method for preparing thermally induced delayed fluorescent semiconductors. The preparation method includes the following steps: the intermediate raw material F1-1 undergoes boronate or borate reaction to obtain intermediate raw materials F1-3, F1-2 and F1 -3 is coupled under catalytic conditions to obtain F1-4, and then undergoes ester hydrolysis to obtain F1-5, and F1-5 undergoes ring-closing reaction to obtain F1-6.
[0066]
[0067] A of each intermediate diagram in the above steps 1 、A 2 , B 1 , B 2 ,Z 1 ~ Z 3 , R 1 ~R 3 , 1, m, n and Y in the general formula (I) 1 ~Y 4 ,Z 1 ~ Z 3 , R 1 ~R 3 , l, m, n each independently have the same limited range, A 3 and B 3 Each independently represents one of chlorine, bromine or iodine, and Ra represents one of methyl, ethyl or tert-butyl.
[0068] When necessary, the A in F1-6 1 、A 2 , B 1 , B 2 F1-7 can be obtained through C-C coupling reaction or C-N coupling react...
Embodiment 1
[0074] The present embodiment provides species having the structure shown in the following formula M1:
[0075]
[0076] The synthetic route of M1:
[0077]
[0078] Synthesis of compound 1: 1,5-dibromo-2,6-dimethylnaphthalene (5g, 15.9mmol), biboronic acid pinacol ester (4.8g, 19mmol), potassium acetate (4.7g, 48mmol) 1 , 1'-bisdiphenylphosphinoferrocenepalladium dichloride (20mg) was placed in a two-necked flask, after flushing nitrogen for three times, 25mL of anhydrous dimethyl sulfoxide was added; the reaction was carried out under nitrogen protection, 80 After reacting at ℃ for 8 hours, after the completion of the reaction, the reaction was cooled to room temperature, poured into water, and suction filtered to obtain a filter cake as a crude product. The crude product was obtained by column chromatography to obtain compound 1 with a mass of 4.31 g. The yield was 75%.
[0079] Synthesis of Compound 2: Put Compound 1 (4g, 11mmol), methyl 2,5-dibromobenzoate (3.55g,...
Embodiment 2
[0084] The present embodiment provides the species with the structure shown in the following formula M2:
[0085]
[0086] The synthetic route of M2:
[0087]
[0088] Synthesis of compound 1: 1,5-dibromo-2,6-dimethylnaphthalene (5g, 15.9mmol), biboronic acid pinacol ester (4.8g, 19mmol), potassium acetate (4.7g, 48mmol) 1 , 1'-bisdiphenylphosphinoferrocenepalladium dichloride (20mg) was placed in a two-necked flask, after flushing nitrogen for three times, 25mL of anhydrous dimethyl sulfoxide was added; the reaction was carried out under nitrogen protection, 80 After reacting at ℃ for 8 hours, after the completion of the reaction, the reaction was cooled to room temperature, poured into water, and suction filtered to obtain a filter cake as a crude product. The crude product was obtained by column chromatography to obtain compound 1 with a mass of 4.31 g. The yield was 75%.
[0089] Synthesis of Compound 2: Put Compound 1 (4g, 11mmol), methyl 2,5-dibromobenzoate (3.55...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com