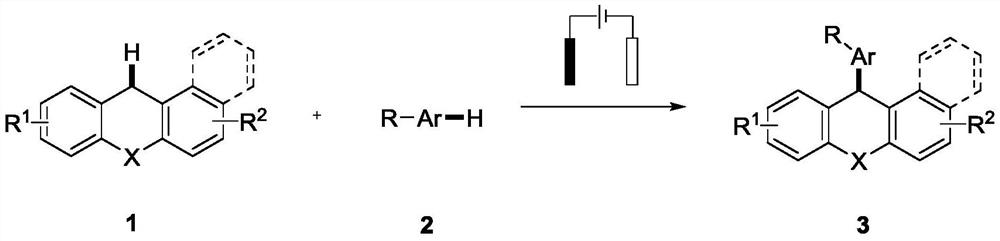
Synthesis method of 9-aryl-9H-oxo/thioxanthene compound
A synthetic method, the technology of thioxanthene, applied in the synthesis of 9-aryl-9H-oxygen/thioxanthene, in the field of organic chemistry, can solve the problem of harsh reaction conditions, non-commercialization of reaction raw materials, and the increase of production costs and products by precious metals The difficulty of purification and other issues, to achieve the effect of mild reaction conditions, high atom economy and step economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Optimization of reaction conditions
[0030] Add xanthene (1a, 32.2mg, 0.5mmol), electrolyte (0.4mol%), anisole (2a, 1.0814g, 10mmol), additive (2eq, 1mmol), organic solvent (5mL) into 10mL without septum in the electrolyzer. Plug the mouth of the bottle with a rubber stopper inserted into the electrode, then insert an empty balloon, connect the potentiostat to the electrode, set the current intensity, pass in the current, and react at 80°C for 12 hours. After the reaction was completed, distilled water (30 mL) was added and extracted with an organic solvent. The organic layers were combined, dried over anhydrous sodium sulfate, and evaporated under reduced pressure with a rotary evaporator to obtain a crude product, which was purified with a silica gel column (petroleum ether / ethyl acetate=30:1) to obtain product 3a. The optimized reaction results are as follows:
[0031]
[0032]
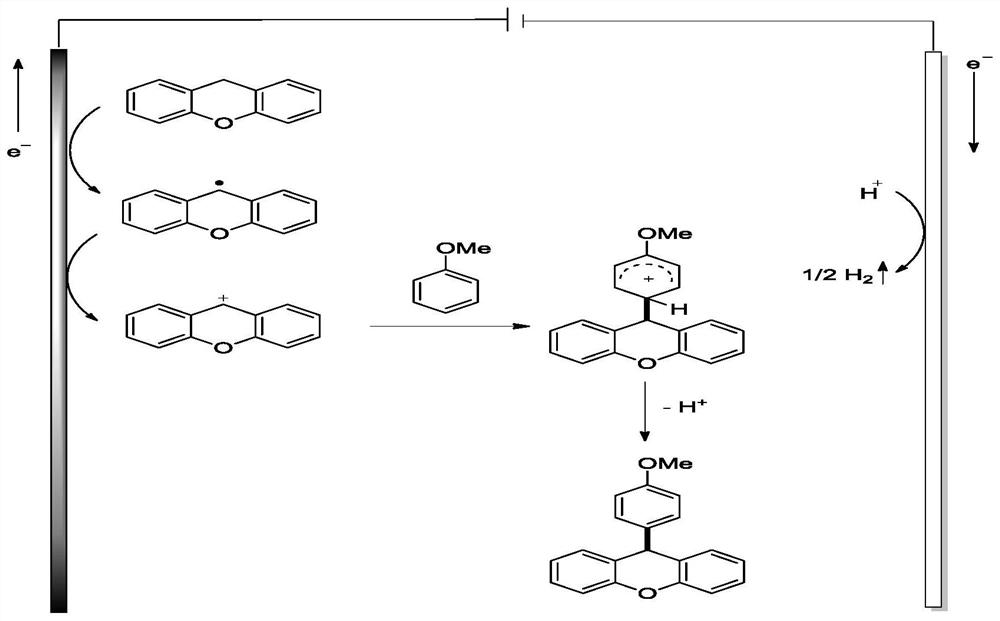
[0033] During the screening of reaction conditions, the additives (label 1-6),...
Embodiment 2
[0035] Xanthene (1a, 91.1mg, 0.5mmol), n-Bu 4 NBF 4 (131.7mg, 0.4mol%), anisole (2a, 1.0814g, 10mmol), methanesulfonic acid (2eq, 96.1mg, 1mmol), 1,2-dichloroethane (5mL) were sequentially added to 10mL without septum in the electrolyzer. Plug the mouth of the bottle with a rubber stopper inserted into the electrode (the carbon rod is used as the anode, and the 1cm×1cm platinum electrode is used as the cathode), then insert an empty balloon, connect the potentiostat to the electrode, set the current intensity to 5mA, and pass in the current. The reaction was carried out at 80° C. for 12 hours. After the reaction was completed, distilled water (30 mL) was added and extracted with ethyl acetate (3×30 mL). The organic layers were combined, dried over anhydrous sodium sulfate, and the crude product was distilled under reduced pressure with a rotary evaporator to obtain a crude product, which was purified by a silica gel column (petroleum ether / ethyl acetate=30:1) to obtain 116....
Embodiment 3
[0038] According to embodiment 2 reaction conditions, only change substrate 1 or substrate 2 structure, obtain reaction product result as follows:
[0039] 9-(3,4-dimethoxyphenyl)-9H-xanthene(3b):Pale yellow solid; 113.6 mg, 71% yield; 1 H NMR (600MHz, CDCl 3 )δ7.22-7.28(m,2H),7.13(dd,J=8.2,1.1Hz,2H),7.06(d,J=7.0Hz,2H),6.98(td,J=7.5,1.2Hz,2H ),6.79(d,J=1.0Hz,2H),6.66(s,1H),5.20(s,1H),3.84(s,3H), 3.76(s,3H). 13 C NMR (151MHz, CDCl 3 )δ151.2, 149.4, 148.0, 139.1, 129.8, 128.0, 124.7, 123.3, 120.8, 116.6, 111.7, 111.2, 56.0, 44.1. HRMS (ESI) calcd.forC 21 h 18 NaO 3 + ([M+Na] + ):341.1148,found:341.1147.
[0040] 2-methyl-4-(9H-xanthen-9-yl)phenol(3c):Pale yellow solid; 74.7mg, 52% yield; 1 H NMR (600MHz, CDCl 3 )δ7.18(t, J=7.5Hz, 2H), 7.11 (d, J=8.1Hz, 2H), 7.04(d, J=7.5Hz, 2H), 6.96(t, J=7.4Hz, 2H) , 6.91(s,1H),6.88(dd,J=8.1,1.5Hz,1H),6.64(d,J=8.1Hz,1H),5.14(s,1H),4.63(s,1H),2.15( s,3H). 13 C NMR (151MHz, CDCl 3 )δ152.6, 151.1, 139.1, 131.1, 129.8, 127.9, 127.2, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com