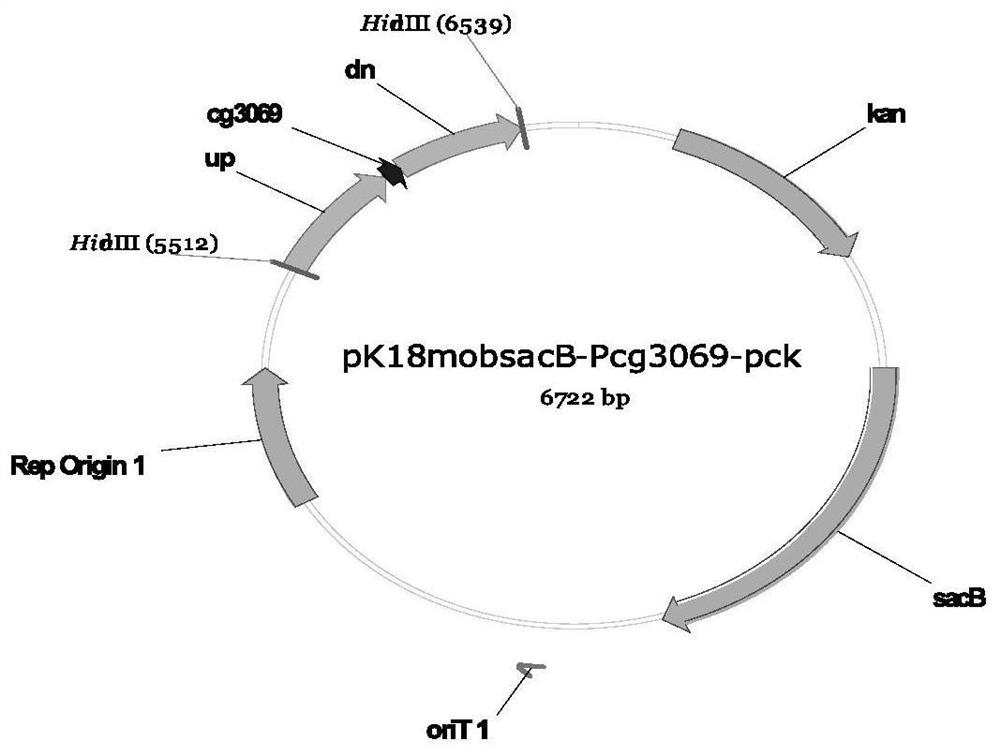
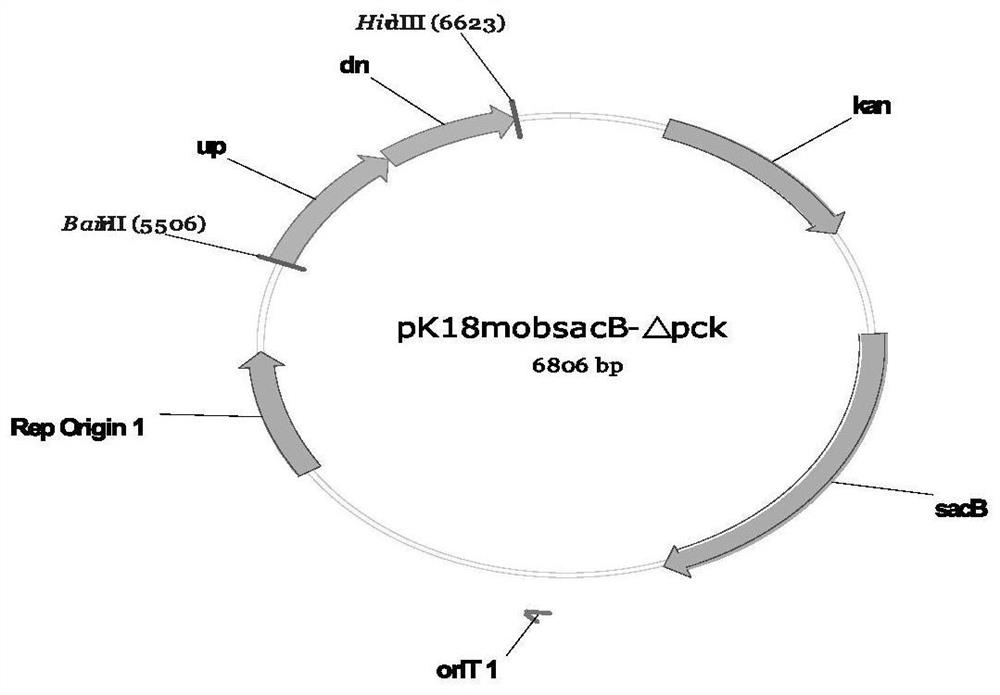
Recombinant corynebacterium glutamicum and method for producing L-threonine
A technology of Corynebacterium glutamicum and threonine, which is applied in the field of bioengineering and can solve the problems of few reports on the synthesis of oxaloacetate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Recombinant plasmid pK18mobsacB-P sod -lysC V1M-T311I Construction of and replacement in ATCC13032
[0070] (1) pK18mobsacB-P sod -lysC V1M-T311I Plasmid construction
[0071] Using the ATCC13032 genome as a template, the upstream homology arm up was obtained by PCR amplification with the P21 / P22 primer pair, and the promoter fragment P was obtained by PCR amplification with the P23 / P24 primer pair sod , PCR amplification with P25 / P26 primer pair to obtain lysC V1M-T311I , PCR amplification with 27 / 28 primer pair to obtain the downstream homology arm dn. Use the P21 / P24 primer pair to up, P sod Perform fusion PCR for the template to obtain the fragment up-P sod . Use the P21 / P28 primer pair to up-P sod , lysC V1M-T311I , dn as a template for fusion PCR to obtain the full-length fragment up-P sod -lysC V1M-T311I -dn. The full-length fragment was digested with BamHI, and pK18mobsacB was digested with the same enzyme. The two digested products were...
Embodiment 2
[0074] Example 2: Recombinant plasmid pK18mobsacB-P cspB -hom G378E Construction of and replacement in SMCT018
[0075] (1) pK18mobsacB-P cspB -hom G378E Plasmid construction
[0076] Using the ATCC13032 genome as a template, the upstream homology arm up was obtained by PCR amplification with the P29 / P30 primer pair, and the promoter fragment P was obtained by PCR amplification with the P31 / P32 primer pair cspB , using P33 / P34 primer pair to carry out PCR amplification to obtain hom G378E , using the P35 / P36 primer pair to perform PCR amplification to obtain the downstream homology arm dn. Use the P29 / P32 primer pair to up, P scpB Perform fusion PCR for the template to obtain the fragment up-P cspB . With P29 / P36 primer pair to up-P cspB 、hom G378E , dn as a template for fusion PCR to obtain the full-length fragment up-P cspB -hom G378E-dn. The full-length fragment was digested with BamHI, and pK18mobsacB was digested with the same enzyme. The two digested produc...
Embodiment 3
[0079] Example 3: Recombinant plasmid pK18mobsacB-P sod -thrC V1M Construction of and replacement in SMCT019
[0080] (1) pK18mobsacB-P sod -thrC V1M Plasmid construction
[0081] Using the ATCC13032 genome as a template, the upstream homology arm up was obtained by PCR amplification with the P37 / P38 primer pair, and the promoter fragment P was obtained by PCR amplification with the P39 / P40 primer pair sod -thrC V1M , dn was obtained by PCR amplification with P41 / P42 primer pair. Use the P37 / P42 primer pair to up, P sod -thrC V1M , dn as a template for fusion PCR to obtain the fragment up-P sod -thrC V1M -dn. The full-length fragment was digested with BamHI, and pK18mobsacB was digested with the same enzyme. The two digested products were connected with T4DNA Ligase, transformed into Trans1 T1 competent cells, and the recombinant plasmid pK18mobsacB-P was obtained sod -thrC V1M .
[0082] (2) ThrC replacement in SMCT019
[0083] SMCT019 competent cells were prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com