Synthetic method of transfluthrin intermediate
A technique of transfluthrin and a synthetic method, which is applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve problems such as low yield, achieve simple reaction, high yield, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
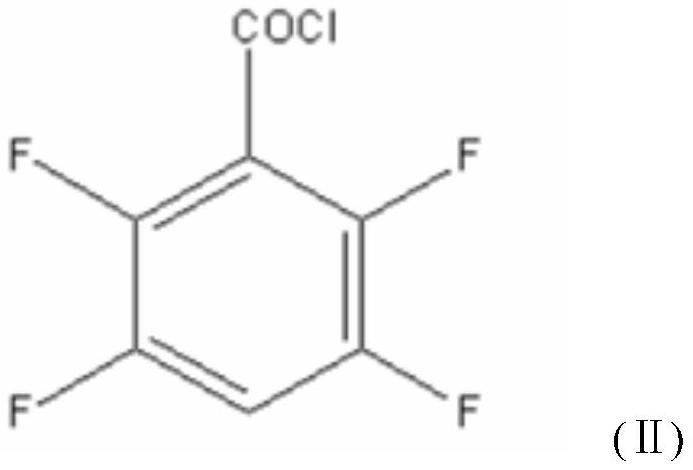
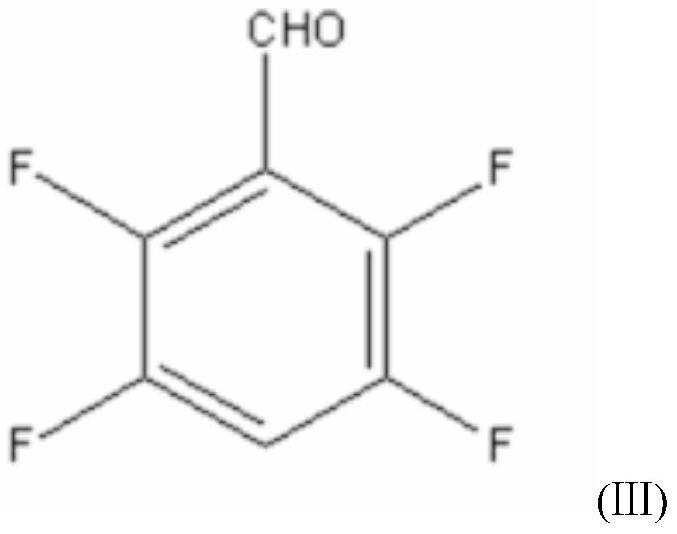
[0048] A new synthetic method of a transfluthrin intermediate 2,3,5,6-tetrafluorobenzyl alcohol, the method consists of the following steps:
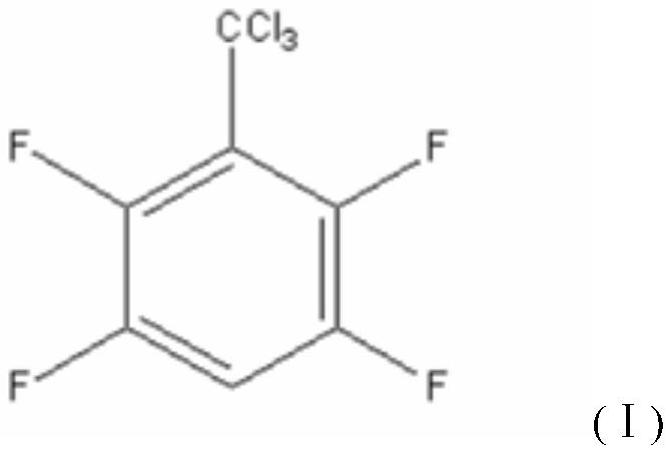
[0049] (1) Synthesis of 2,3,5,6-tetrafluorotrichloromethylbenzene
[0050] In a 500mL four-necked flask with a stirring rod, a reflux condenser, a thermometer and a constant pressure dropping funnel, 92.4g (0.6mol) of carbon tetrachloride, 20.2g (0.15mol) of aluminum chloride and 5.0 zeolite were charged. g, after heating to 70°C, 9 g (0.06 mol) of 2,3,5,6-tetrafluorobenzene was slowly added dropwise, and the dropwise addition was completed within 1 hour. After the dropwise addition, the constant temperature reaction was continued for 1 h, and 300 mL of ice water was added to the reaction system after cooling. After the solid was filtered out, the organic layer was separated, and the aqueous layer was extracted three times with 50 mL of carbon tetrachloride, and the organic layer was combined, washed with 5% aqueous sodium bicarbonate ...
Embodiment 2
[0058] A new synthetic method of a transfluthrin intermediate 2,3,5,6-tetrafluorobenzyl alcohol, the method consists of the following steps:
[0059] (1) Synthesis of 2,3,5,6-tetrafluorotrichloromethylbenzene
[0060] In a 500mL four-necked flask with a stirring rod, a reflux condenser, a thermometer and a constant pressure dropping funnel, 138.6g (0.9mol) of carbon tetrachloride, 20.2g (0.15mol) of aluminum chloride and 5.0 zeolite were charged. g, after heating to 70°C, 9 g (0.06 mol) of 2,3,5,6-tetrafluorobenzene was slowly added dropwise, and the dropwise addition was completed within 1 hour. After the dropwise addition, the constant temperature reaction was continued for 1 h, and 300 mL of ice water was added to the reaction system after cooling. After the solid was filtered out, the organic layer was separated, and the aqueous layer was extracted three times with 50 mL of carbon tetrachloride, and the organic layer was combined, washed with 5% aqueous sodium bicarbonate...
Embodiment 3
[0068] A new synthetic method of a transfluthrin intermediate 2,3,5,6-tetrafluorobenzyl alcohol, the method consists of the following steps:
[0069] (1) Synthesis of 2,3,5,6-tetrafluorotrichloromethylbenzene
[0070] In a 500mL four-necked flask with a stirring rod, a reflux condenser, a thermometer and a constant pressure dropping funnel, 46.2g (0.3mol) of carbon tetrachloride, 20.2g (0.15mol) of aluminum chloride and 5.0 zeolite were charged. g, after heating to 70°C, 9 g (0.06 mol) of 2,3,5,6-tetrafluorobenzene was slowly added dropwise, and the dropwise addition was completed within 1 hour. After the dropwise addition, the constant temperature reaction was continued for 1 h, and 300 mL of ice water was added to the reaction system after cooling. After the solid was filtered out, the organic layer was separated, and the aqueous layer was extracted three times with 50 mL of carbon tetrachloride, and the organic layer was combined, washed with 5% aqueous sodium bicarbonate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com