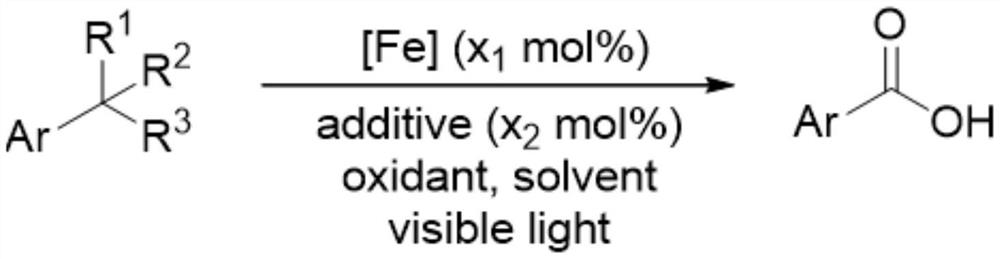
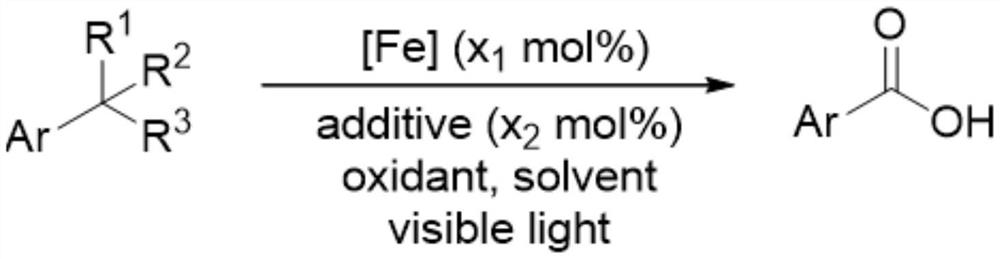
Method for preparing aromatic acid through oxidation reaction of 1, 2, 3-grade alkyl substituted aromatic compounds under iron catalysis
An oxidation reaction and iron compound technology, which is applied in the preparation of organic compounds, chemical instruments and methods, and preparation of sulfonic acid, can solve the problems of serious pollution and inability to oxidize, and achieve simple operation, mild oxidation conditions, and substrate applicability broad effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add the iron catalyst (x 1 mol%), additives (x 2 mol%), toluene (2mmol), anhydrous acetonitrile (4 milliliters), after the addition finishes, stir and dissolve, mix uniformly, place the reaction tube under the light (hv) of wavelength 390nm to irradiate and constantly stir, treat to react after finishing The reaction tube was removed from the light source, the reaction mixture was transferred to a flask, the crude product was obtained by distillation under reduced pressure, and 190.7 mg of benzoic acid was obtained by flash column chromatography with a yield of 78%. The product was a white solid. 1 HNMR (400MHz, CDCl 3 )δ12.25(brs,1H,-COOH),8.15(d,J=7.4Hz,2H,Ar-H),7.63(t,J=7.4Hz,1H,Ar-H),7.49(t,J =7.5Hz,2H,Ar-H). 13 C NMR (101MHz, CDCl 3 )δ172.7, 133.8, 130.2, 129.3, 128.5.
Embodiment 2
[0047] By the method described in Example 1, the difference is that the amount of reagent used is: iron catalyst (x 1 mol%), additives (x 2 mol%), 4-cyanotoluene (46.9 mg, 0.4 mmol), anhydrous acetonitrile (4 ml); the reaction tube was heated to 50° C. to obtain 50.4 mg of 4-cyanobenzoic acid with a yield of 86%. 1 H NMR (400MHz, DMSO-d 6 )δ13.58(s,1H,-COOH),8.07(d,J=8.3Hz,2H,Ar-H),7.97(d,J=8.3Hz,2H,Ar-H). 13 C NMR (101MHz, DMSO-d 6 )δ166.1, 134.8, 132.7, 130.0, 118.2, 115.1.IRν(neat, cm- 1 )3418,2987,1700,1653,1430,1323,1288,1131,768,749.
Embodiment 3
[0049] By the method described in Example 1, the difference is that the amount of reagent used is: iron catalyst (x 1 mol%), additives (x 2 mol%), 4-fluorotoluene (44.1 mg, 44 microliters, 0.4 mmol), anhydrous acetonitrile (4 milliliters); irradiation under the light (hv) of wavelength 350nm, obtain 34.4 milligrams of 4-fluorobenzoic acid, productive rate 61%. 1 H NMR (400MHz, DMSO-d 6 )δ13.07(brs,1H,-COOH),8.00(dd,J 1 =8.7,J 2 =5.7Hz, 2H, Ar-H), 7.31(t, J=8.9Hz, 2H, Ar-H). 13 C NMR (101MHz, DMSO-d 6 )δ166.4,165.0(C-F,d, 1 J C-F =252.5Hz), 132.1(C-F,d, 3 J C-F =9.5Hz), 127.4(C-F,d, 4 J C-F =2.8Hz), 115.7(C-F,d, 2 J C-F =30.3Hz). 19 F NMR (376MHz, DMSO-d 6 )δ-106.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com