Preparation method and application of non-fluorescent organic small molecule compound and pentamethine cyanine dye
A small molecule compound, non-fluorescence technology, applied in the direction of organic dyes, methine-based/polymethine-based dyes, organic chemistry, etc., which can solve the limitations of sensitivity, stability, background fluorescence, space/time controllable, and fluorescence turn-on controllable and other problems to achieve the effect of simplifying the operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Photo-induced preparation of pentamethine dyes: 2-thiophene-Cy5 indole compounds with amino side chain structure
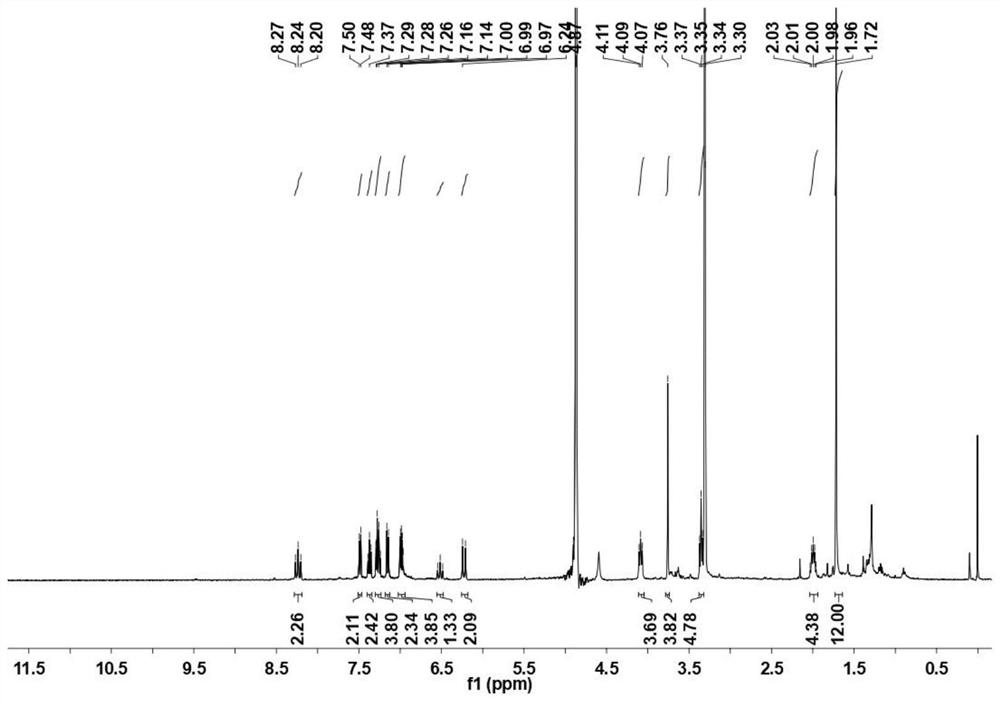
[0037] (1) Add 1-propylamine-2,3,3-trimethylindole (75.6mg), 2-thiophene-succinimide active ester (23.9mg) into a 25mL single-necked flask, and then add 5mLCHCl 3 , 56 μL triethylamine, ultrasonically dissolve the solid completely, heat to 65°C, use white LED light to illuminate, reflux the mixture for 12h, then cool to room temperature, spin the organic solvent to dry, add dichloromethane and neutral alumina dry method The sample was mixed, and the product was further separated and purified by neutral alumina chromatography (the eluent ratio was dichloromethane:methanol=50:1). The reaction yield was 8%, and 3.2 mg of 2-thiopheneB-Cy5 was obtained. figure 1 For the synthetic pentamethine cyanine dye fluorescent molecule (2-thiophene B-Cy5) in embodiment 1 step (1) 1 H-NMR spectrum. 1 HNMR (400MHz, CDCl 3 δ): 8.33(t, J=5.2Hz, 2H), 7.74(t, J=13.0Hz, 2H), ...
Embodiment 2
[0040] Photoinduced Preparation of Pentamethine Dye: Benzene-Cy5
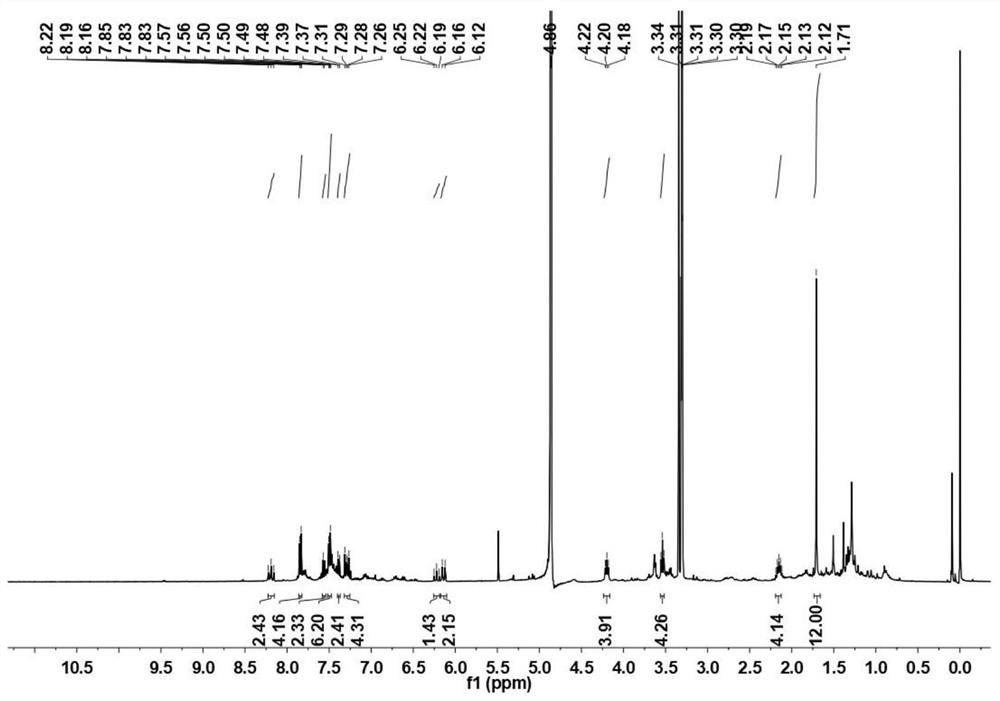
[0041] (1) Add 1-propylamine-2,3,3-trimethylindole (75.6mg), benzyl-succinimide active ester (21.9mg) into a 25mL one-necked flask, then add 5mLCHCl 3 , 56 μL triethylamine, ultrasonically dissolve the solid completely, heat to 65°C, use white LED light to illuminate, reflux the mixture for 12h, then cool to room temperature, spin the organic solvent to dry, add dichloromethane and neutral alumina dry method The sample was mixed, and the product was further separated and purified by neutral alumina chromatography (the eluent ratio was dichloromethane:methanol=50:1). The reaction yield was 6%, and 2.3 mg of benzyl-Cy5 was obtained. image 3For the synthetic pentamethine cyanine dye fluorescent molecule (benzyl-Cy5) in embodiment 2 step (1) 1 H-NMR spectrum. 1 H NMR (400MHz, MeODδ): 8.19(t, J=3.1Hz, 2H), 7.87-7.83(m, 4H), 7.58-7.54(m, 2H), 7.51-7.46(m, 6H), 7.38(dd , J=6.5, 5.5Hz, 2H), 7.29(dd, J=12.2, 7.7Hz,...
Embodiment 3
[0043] Photoinduced Preparation of Pentamethine Dye: 3-Indoleethyl-Cy5
[0044] (1) Add 1-propylamine-2,3,3-trimethylindole (75.6mg), 3-indoleethyl-succinimide active ester (27.2mg) into a 25mL single-necked flask, then add 5mLCHCl 3 , 56 μL triethylamine, ultrasonically dissolve the solid completely, heat to 65°C, use white LED light to illuminate, reflux the mixture for 12h, then cool to room temperature, spin the organic solvent to dry, add dichloromethane and neutral alumina dry method The sample was mixed, and the product was further separated and purified by neutral alumina chromatography (the eluent ratio was dichloromethane:methanol=50:1). The reaction yield was 6%, and 2.6 mg of 3-indoleethyl-Cy5 was obtained. Figure 5 For the synthetic pentamethine cyanine dye fluorescent molecule (3-indole ethyl-Cy5) in embodiment 2 step (1) 1 H-NMR spectrum. 1 H NMR (400MHz, MeODδ): 8.16-8.03(m, 2H), 7.52(t, J=7.9Hz, 2H), 7.38-7.33(m, 2H), 7.27(t, J=9.1Hz, 3H), 7.14(dt, J=10.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com