Near-infrared two-region dye based on naphthalimide derivative as well as preparation and application of near-infrared two-region dye
A naphthalene diimide, near-infrared technology, applied in the field of nano-biomedical imaging materials, can solve the problems of fluorescence quenching, low tissue penetration depth, photobleaching, etc., to increase the variety and selection space, synthesis route and preparation simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The structure of the small molecule in the second near-infrared region provided by this example is as follows:
[0041]
[0042] For the preparation method of this embodiment, see Figure 8 As shown, it specifically includes the following steps:
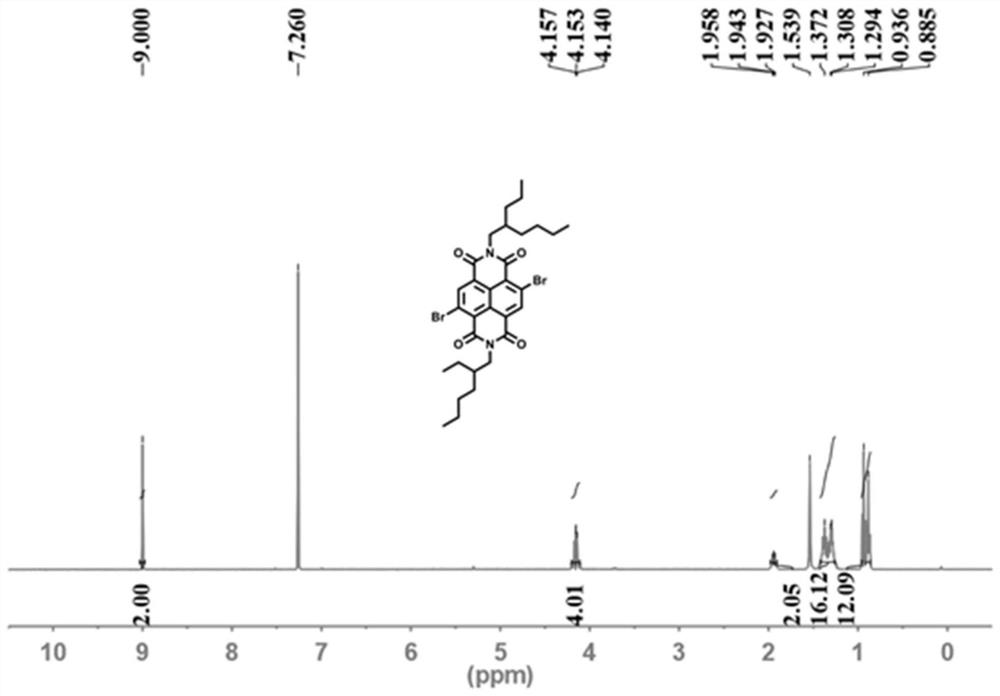
[0043] 1) Add 5g 1,4,5,8-naphthalene tetracarboxylic dianhydride (18.64mmol, 1equiv) and 75mL oleum in a 250mL round bottom flask, add catalytic amount of iodine to the reaction system, and then 1.72 mL of bromine (33.52 mmol, 1.8 equiv) was added slowly under low temperature, then heated to 50° C. and the reaction was stirred for 48 hours. After cooling, slowly add a saturated solution of sodium thiosulfate to remove excess bromine, then pour the reaction solution into ice water to form a yellow precipitate, filter and wash with water to obtain a bright yellow solid, which is 2,6-dibromo-1,4 , 5,8-naphthalene tetracarboxylic dianhydride, directly proceed to the next step reaction.
[0044] 2) Add 2g of 2,6-dibromo-1,4,5...
Embodiment 2
[0048] Small molecule contrast agent in the second near-infrared region
[0049] Dissolve 1 mg of the small molecule in the second near-infrared region (prepared in Example 1) in 1 mL of dichloromethane, and dissolve 6 mg of the amphiphilic lipid molecule DSPE-PEG5000 in 1 mL of aqueous solution, and mix the prepared dichloromethane solution and aqueous solution to obtain a mixed liquid , and then stirred overnight at room temperature to evaporate excess dichloromethane to obtain a small-molecule contrast agent in the second near-infrared region.
[0050] The obtained small molecule contrast agent in the second near-infrared region was characterized by transmission electron microscopy, and the results are as follows: Figure 5 As shown, the morphology is spherical and the size is about 50nm.
Embodiment 3
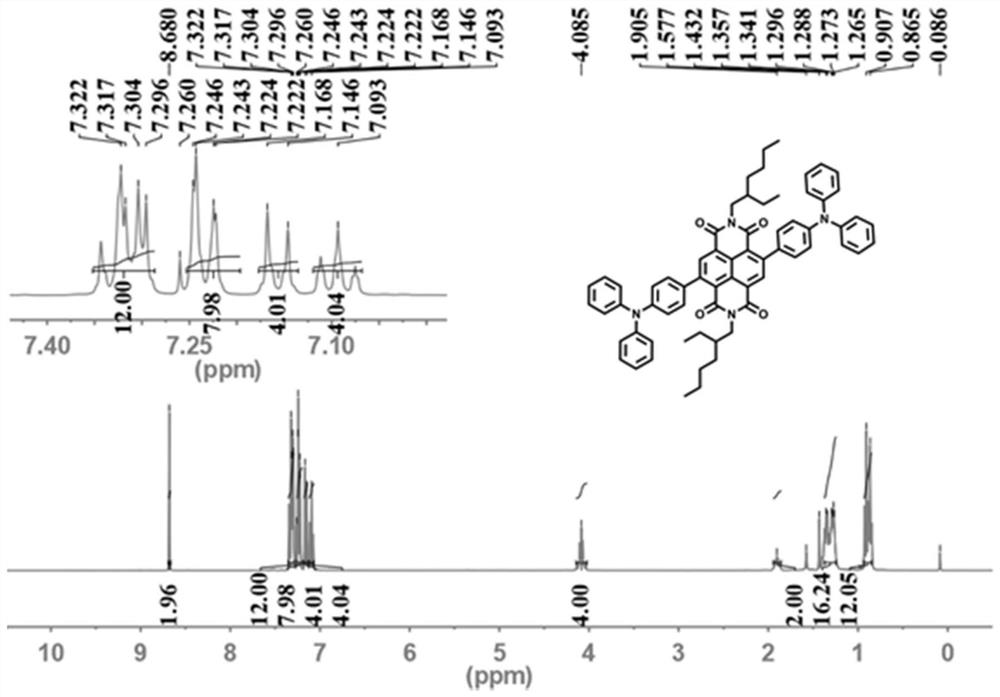
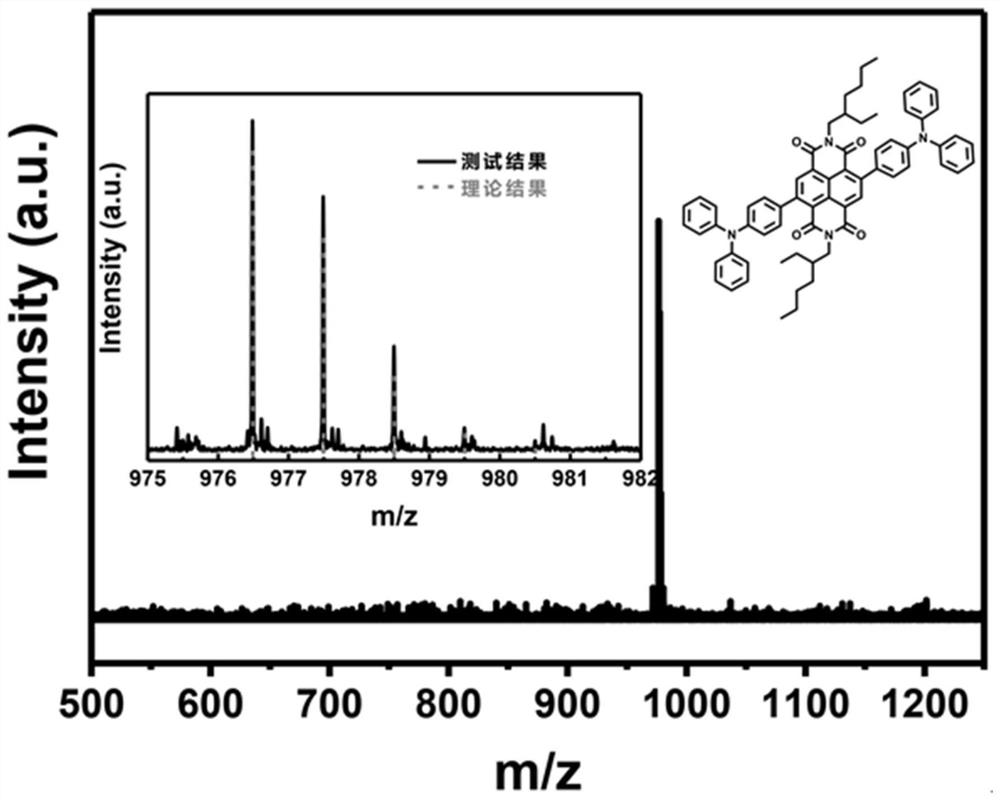
[0059] Compared with Example 1, most of them are the same, except adjusting the consumption of 4-(diphenylamino) phenylboronic acid pinacol ester, Aliquat 336, potassium carbonate, tetrakis (triphenylphosphine) palladium, so that N, N'-(2'-Ethyl)hexyl-2,6-dibromo-1,4,5,8-naphthalene tetracarboxylic acid diimide, 4-(diphenylamino)phenylboronic acid pinacol ester, Aliquat 336, potassium carbonate, and tetrakis(triphenylphosphine)palladium are added in a ratio of 1 mmol: 2.1 mmol: 10 mg: 5 mmol: 0.05 mmol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com