Application of Shuanghuanglian preparation in virus infection resistance
A coronavirus, a technology for use, applied in the field of medicine, can solve the problems of limited effect of coronavirus, lack of effective treatment of acute viral infectious diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
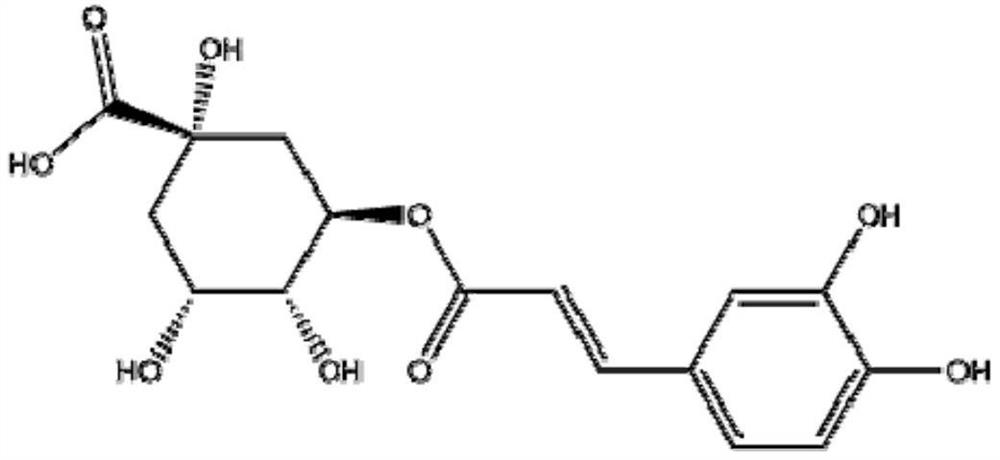
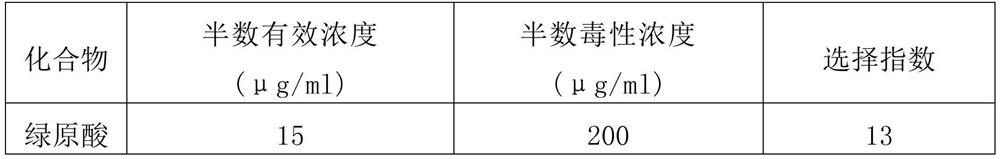
[0077] Embodiment 1. Chlorogenic acid is to the inhibitory effect of SARS virus activity
[0078] In this example, through anti-SARS virus (virus strain: BJ-01) activity screening (virus-cell level model), it is verified whether chlorogenic acid has an inhibitory effect on SARS virus.
[0079] Test principle: Vero-E6 cells are used as virus host cells (susceptible cells) to test the effect of samples against virus-infected cells. The detection indicators are cell degeneration (CPE) and the protection rate of infected cells.
[0080] testing method:
[0081] Seed Vero-E6 cells in a 96-well culture plate at 37°C, 5% CO 2 Cultivate, add samples and SARS virus of different dilution concentrations respectively, establish virus control, cell control and sample control. Observe the results under the microscope every day, record the CPE, and measure the OD value with neutral red staining, and calculate and evaluate the anti-SARS virus activity of the sample with reference to the con...
Embodiment 2
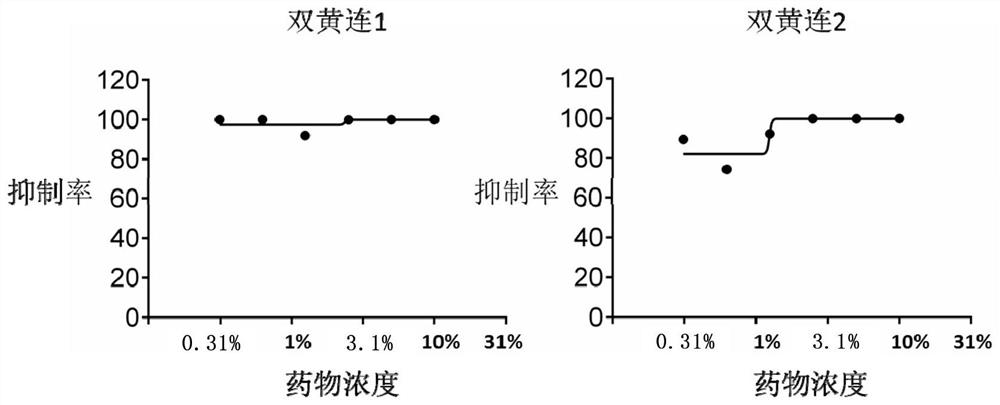
[0085] Example 2. The inhibitory effect of Shuanghuanglian preparation (oral solution) on avian influenza virus.
[0086] In this example, the anti-influenza virus activity screening (neuraminidase activity inhibition test) was used to test the inhibitory effect of Shuanghuanglian preparation (oral solution) on avian influenza virus.
[0087] Virus strains: Avian influenza virus strain H5N1 (2004), Tamiflu-resistant influenza virus strain H1N1
[0088] Shuanghuanglian preparation (oral solution): commercially available.
[0089] Test principle:
[0090] Neuraminidase (NA) is an important glycoprotein on the envelope of influenza virus, which plays an important role in the replication and transmission of influenza virus. Inhibitors of influenza virus neuraminidase (NA) are the targets of Tamiflu, a commonly used clinical anti-influenza virus drug. Using influenza virus neuraminidase as an active drug screening and evaluation target can efficiently and rapidly screen and eval...
Embodiment 3
[0100] Inhibitory effect of Shuanghuanglian preparation (oral solution) on MERS-CoV virus activity
[0101] In this example, through the anti-MERS-CoV pseudovirus invasion activity screening (virus-cell level model), it was verified whether the Shuanghuanglian preparation (oral solution) had an inhibitory effect on the MERS-CoV virus.
[0102] Test principle: Huh7 cells are used as virus host cells (susceptible cells), and the test sample blocks the activity of MERS-CoV membrane protein modified pseudovirus-infected cells, which can reflect the antiviral activity of the sample to interfere with the key target of MERS-CoV infection . The detection index is the activity level of the reporter gene on the pseudovirus genome.
[0103] testing method:
[0104] Huh7 cells were inoculated in 96-well culture plates one day in advance, and the activity plate and cytotoxicity plate were respectively set up, and cultured at 37°C and 5% CO2. The activity assay plate and the cytotoxicity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com