Preparation method of bacteriostatic hydrogel containing human umbilical cord mesenchymal stem cell freeze-dried powder
A stem cell, human umbilical cord technology, applied in bandages, medical science and other directions, can solve the problems of not promoting rapid wound healing, poor bacteriostatic effect, etc., achieving good water absorption and swelling performance, preventing infection, and promoting the effect of accelerating healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
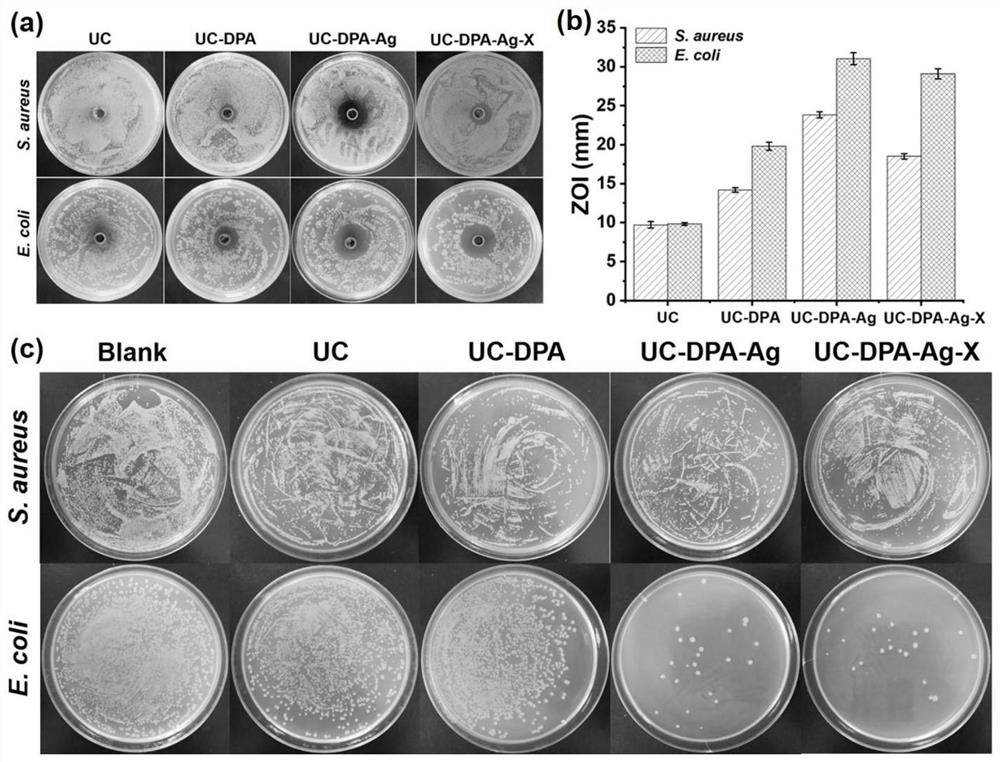
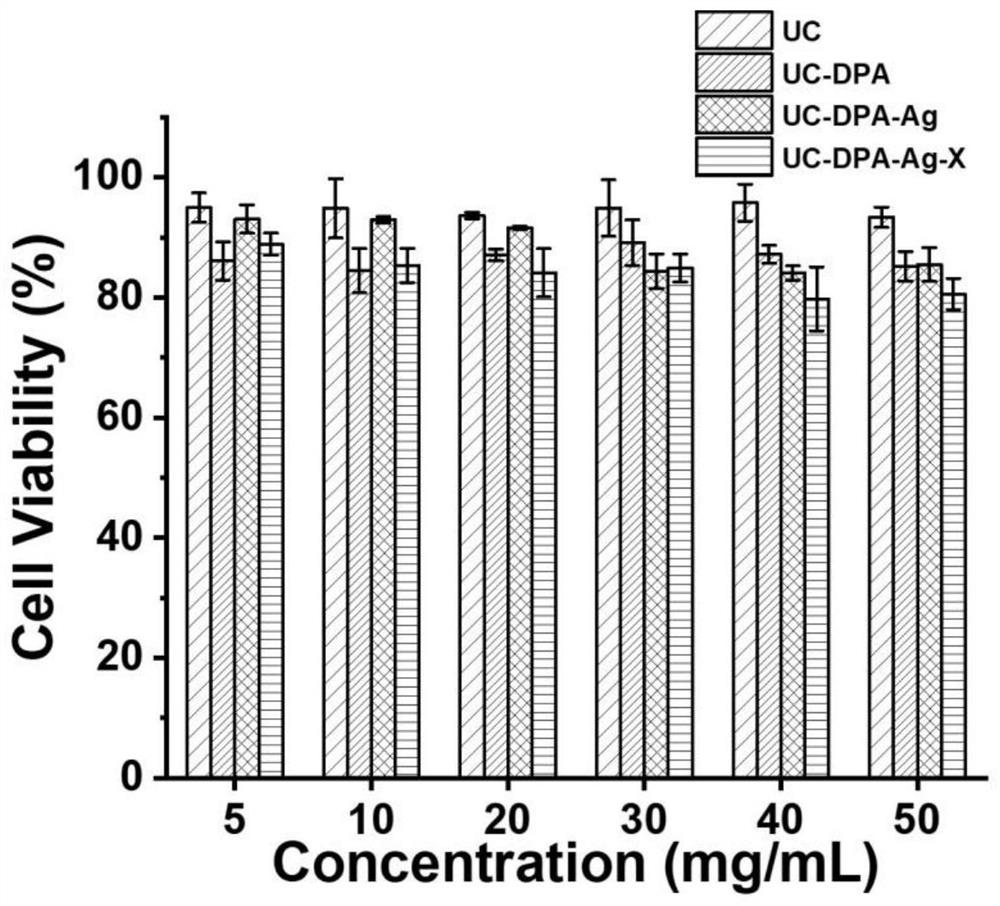
[0033] A preparation method of the antibacterial hydrogel containing human umbilical cord mesenchymal stem cell freeze-dried powder of the present invention comprises the following steps:
[0034] Step 1: Human umbilical cord mesenchymal stem cells (human umbilical cords were provided by women who gave birth in Shanghai Dongfang Hospital, China. All subjects were informed and agreed. Human umbilical cord mesenchymal stem cells were isolated, cultured and certified) were cultured in MEM (Gibco, C12571500BT, China) containing 5% Ultra GRo-Advanced cell nutrient supplement (HeliosBioscience, HPCFDCGL50, USA), and incubated in an incubator at 37°C and 5% carbon dioxide. When the density of human umbilical cord mesenchymal stem cells reaches about 80%, wash 3 times with 0.9%wt% NaCl solution, culture in MEM medium without platelet lysis for 24h, collect the supernatant, vacuum at 0.02mbar and- Freeze at 90° C. for 48 hours to obtain freeze-dried powder of human umbilical cord mesen...
Embodiment 1
[0044] The preparation method of the antibacterial hydrogel of the present embodiment adopts following numerical preparation:
[0045] (1) Take by weighing 0.3g chitosan, be dissolved in the 3mL acetic acid solution of pH=4, obtain chitosan solution;
[0046] (2) Weigh 0.3 g of dialdehyde-derived ultrusan, and dissolve it in 6 mL of pure water to obtain a dialdehyde-derived ultrusan solution;
[0047] (3) Weigh 0.15g of dopamine hydrochloride and dissolve it in 3mL of pure water to obtain dopamine hydrochloride solution;
[0048] (4) above-mentioned three kinds of solutions are mixed and stirred overnight at room temperature, obtain bisaldehyde ulvasan / chitosan / dopamine hydrogel;
[0049] (5) Weigh 0.3997g (2mM) of silver nitrate solid, dissolve it in 50mL of pure water to obtain a 0.04M silver nitrate solution, and soak the above-mentioned dialdehyde-formed ulvato / chitosan / dopamine hydrogel In the prepared silver nitrate solution, react in a dark environment for 20 minutes ...
Embodiment 2
[0052] The preparation method of the antibacterial hydrogel of the present embodiment adopts following numerical preparation:
[0053] (1) Take by weighing 0.2g chitosan, be dissolved in the 3mL acetic acid solution of pH=5, obtain chitosan solution;
[0054] (2) Weigh 0.3 g of dialdehyde-derived ultrusan, and dissolve it in 6 mL of pure water to obtain a dialdehyde-derived ultrusan solution;
[0055] (3) Weigh 0.15g of dopamine hydrochloride and dissolve it in 3mL of pure water to obtain dopamine hydrochloride solution;
[0056] (4) above-mentioned three kinds of solutions are mixed and stirred overnight at room temperature, obtain bisaldehyde ulvasan / chitosan / dopamine hydrogel;
[0057] (5) Weigh 0.7994g (4mM) of silver nitrate solid and dissolve it in 50mL of pure water to obtain a 0.08M silver nitrate solution. Soak the above-mentioned dialdehyde-derived ultrusan / chitosan / dopamine hydrogel in the configured silver nitrate solution, and react in a dark environment for 20 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com