Fermentation and purification process of helicobacter pylori LuxS hexamer recombinant protein
A technology of Helicobacter pylori and fermentation process, applied in the directions of fermentation, recombinant DNA technology, bacterial peptides, etc., can solve the problems of complicated process, difficult stability of oral Helicobacter pylori vaccine, high cost, and achieve simple and pure fermentation and purification process. High, immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
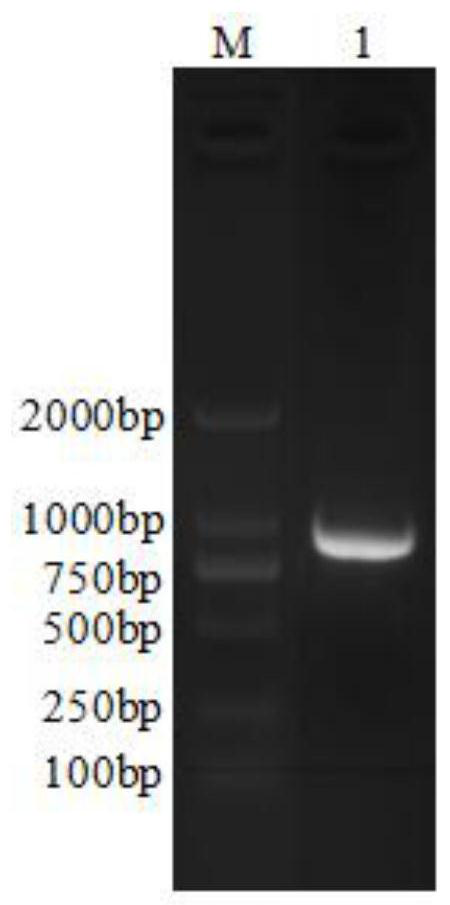
[0069] Example 1 LTA1-LuxS-LTA1-LTA2-LTB gene construction
[0070] (1) LTA1, LuxS, LTA2, LTB gene cloning and connection
[0071] 1. LTA1, LTA2, and LTB genes are derived from LT, and LT is derived from whole gene synthesis (Shanghai Jierui Bioengineering Co., Ltd.).
[0072] 2. The LuxS gene is derived from Helicobacter pylori SS1 strain.
[0073] 3. According to the principle of primer design, design corresponding primers and add enzyme cutting sites. Primer sequences are shown in Table 1:
[0074] Table 1
[0075]
[0076]
[0077] 4. LTA1, LuxS, LTA2, LTB gene connection
[0078] (1) The high-fidelity PCR method is used to amplify the gene sequence, and the high-fidelity system and procedures refer to the instructions.
[0079] (2) High-fidelity PCR was performed with LT as template and F-82 and R-82a as primers.
[0080] (3) High-fidelity PCR was performed with the whole genome of Helicobacter pylori SS1 strain as template and F-83a and R-83a as primers.
[...
Embodiment 2
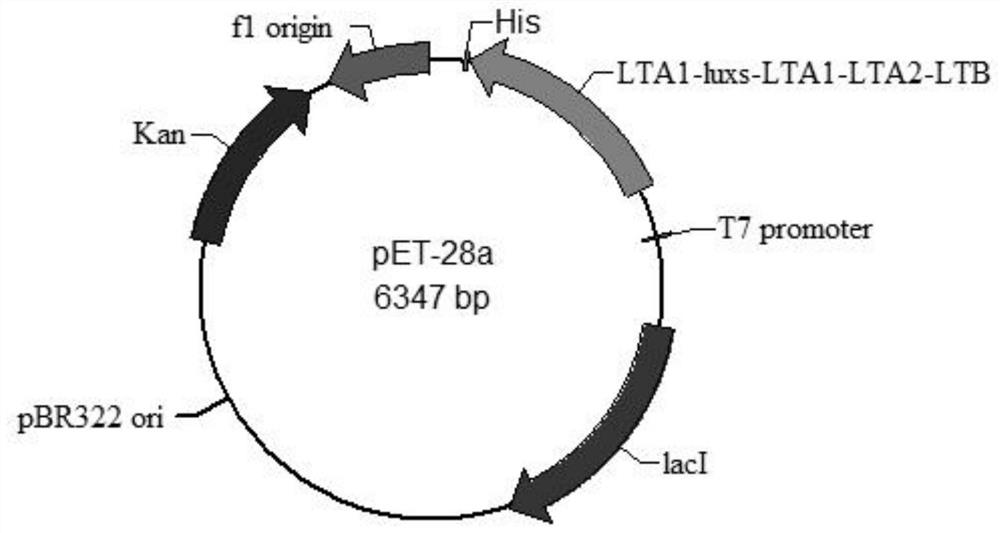
[0113] Embodiment 2, the fermentation process of pET28a-LTA1-LuxS-LTA1-LTA2-LTB Escherichia coli engineering bacteria
[0114] (1) Basic procedure of fermentation
[0115] 1. Inoculation and culture of pET28a-LTA1-LuxS-LTA1-LTA2-LTB Escherichia coli
[0116] The constructed pET28a-LTA1-luxs-LTA1-LTA2-LTB Escherichia coli engineered bacteria (culture 4) was taken out from the -80°C ultra-low temperature refrigerator, and the engineered bacteria were inoculated in LB liquid medium containing 0.001% kanamycin Medium, 37°C, 220rpm constant temperature culture overnight.
[0117] 2. pET28a-LTA1-luxs-LTA1-LTA2-LTB Escherichia coli transferred to 10L fermenter culture
[0118] Take out the engineered bacteria cultivated overnight and inoculate in a 10L fermenter with a 10% inoculation ratio, the fermentation medium is TB medium, and the inoculation volume is 5L. Incubate for 8 hours at 37°C with a dissolved oxygen concentration of 30%. The composition of TB medium is: potassium d...
Embodiment 3
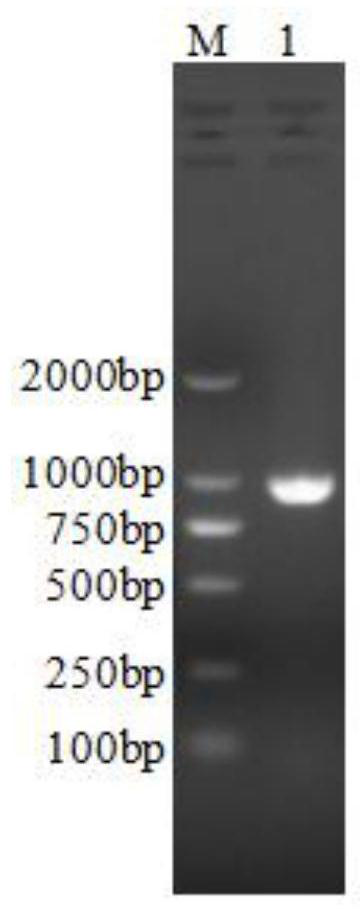
[0152] Embodiment 3 Purification process of LuxS hexamer recombinant protein
[0153] (1) Basic conditions for purification
[0154] Instrument system: AKTAprimer (GE)
[0155] Filler: D-Galactose
[0156] Purification column model: 7510081 (Bio-Rad)
[0157] Packing volume: 2mL
[0158] Buffer C1 composition: disodium edetate 0.11%, sodium chloride 1.17%, Tris 0.30%, glycerol 1.00%.
[0159] Buffer C2 composition: 0.11% disodium edetate, 1.17% sodium chloride, 0.30% Tris, 1.00% glycerol, 4.50% D-galactose.
[0160] (2) Purification process
[0161] 1. Bacteria
[0162] Take 10 g of the bacteria obtained from the fermentation, add buffer C1 at a mass (g): volume (mL) ratio of 1:20, and use a shearer to shear and suspend the buffer containing the bacteria at 4°C.
[0163] Use RO water to flush the high-pressure homogenizer (AH-1500, ATS Industrial Systems Co., Ltd.) pipeline. Turn on the low-temperature refrigeration system for pre-cooling and reserve. Add the pre-cool...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com