Clindamycin phosphate injection preparation and preparation method thereof
A clindamycin phosphate and injection technology, which is applied in the field of medical invention, can solve the problems of not being able to use children's intramuscular injection, insufficient nitrogen filling in production, and low production efficiency, so as to improve the efficiency of liquid preparation and the stability of liquid medicine , good pH value, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A clindamycin phosphate injection preparation, in every 1000ml injection, comprising:
[0049] Clindamycin Phosphate 130g, Thioglycerol 0.3g, Sodium Hydroxide 8g.
[0050] The preparation method of described clindamycin phosphate injection preparation, comprises the following steps:
[0051] (1) Get sodium hydroxide and dissolve it with 30°C water for injection to prepare a 10% sodium hydroxide solution;
[0052] (2) Add water for injection at 10°C into the liquid mixing tank, add thioglycerol, stir at 100 rpm for 40 minutes until completely dissolved, and make a thioglycerol solution;
[0053] (3) Get clindamycin phosphate and 10% sodium hydroxide solution and add them to the thioglycerol solution in 8 times;
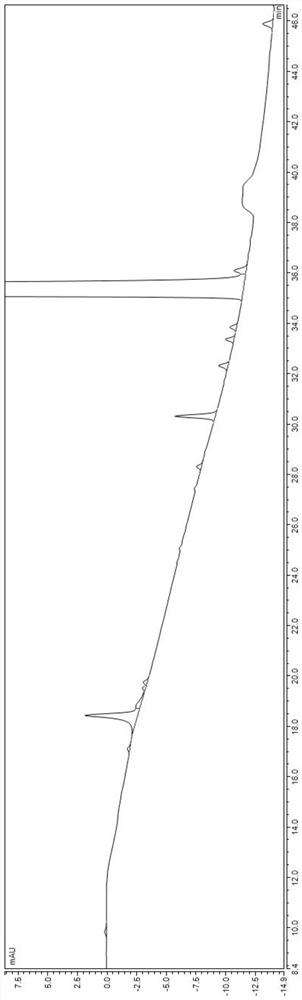
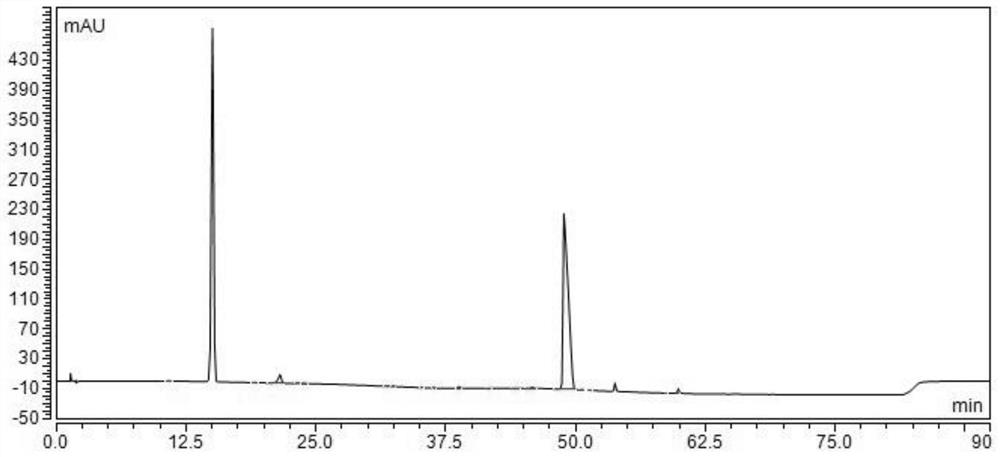
[0054] (4) Dilute to 1000ml with water for injection, stir until the solution is evenly mixed, check the pH value, first filter once through a filter membrane with a pore size of 0.45 μm, and then filter twice through a filter membrane with a pore size of 0.22...
Embodiment 2
[0059] A clindamycin phosphate injection preparation, in every 1000ml injection, comprising:
[0060] Clindamycin Phosphate 170g, Thioglycerol 0.8g, Sodium Hydroxide 16g.
[0061] The preparation method of described clindamycin phosphate injection preparation, comprises the following steps:
[0062] (1) Get sodium hydroxide and dissolve it with water for injection at 10°C to prepare a 10% sodium hydroxide solution;
[0063] (2) Add water for injection at 30°C into the liquid mixing tank, add thioglycerol, stir at 150 rpm for 30 minutes until completely dissolved, and make a thioglycerol solution;
[0064] (3) Get clindamycin phosphate and 10% sodium hydroxide solution and add them to the thioglycerol solution in 8 times;
[0065] (4) Dilute to 1000ml with water for injection, stir until the solution is evenly mixed, check the pH value, first filter once through a filter membrane with a pore size of 0.45 μm, and then filter twice through a filter membrane with a pore size of ...
Embodiment 3
[0070] A clindamycin phosphate injection preparation, in every 1000ml injection, comprising:
[0071] Clindamycin Phosphate 150g, Thioglycerol 0.5g, Sodium Hydroxide 12g.
[0072] The preparation method of described clindamycin phosphate injection preparation, comprises the following steps:
[0073] (1) Get sodium hydroxide, dissolve and be mixed with the sodium hydroxide solution that concentration is 10% with the water for injection of 20 ℃;
[0074] (2) Add water for injection at 20°C into the liquid mixing tank, add thioglycerol, stir at 150 rpm for 60 minutes until completely dissolved, and make a thioglycerol solution;
[0075] (3) Get clindamycin phosphate and 10% sodium hydroxide solution and add them to the thioglycerol solution in 8 times;
[0076] (4) Dilute to 1000ml with water for injection, stir until the solution is evenly mixed, check the pH value, first filter once through a filter membrane with a pore size of 0.45 μm, and then filter twice through a filter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com