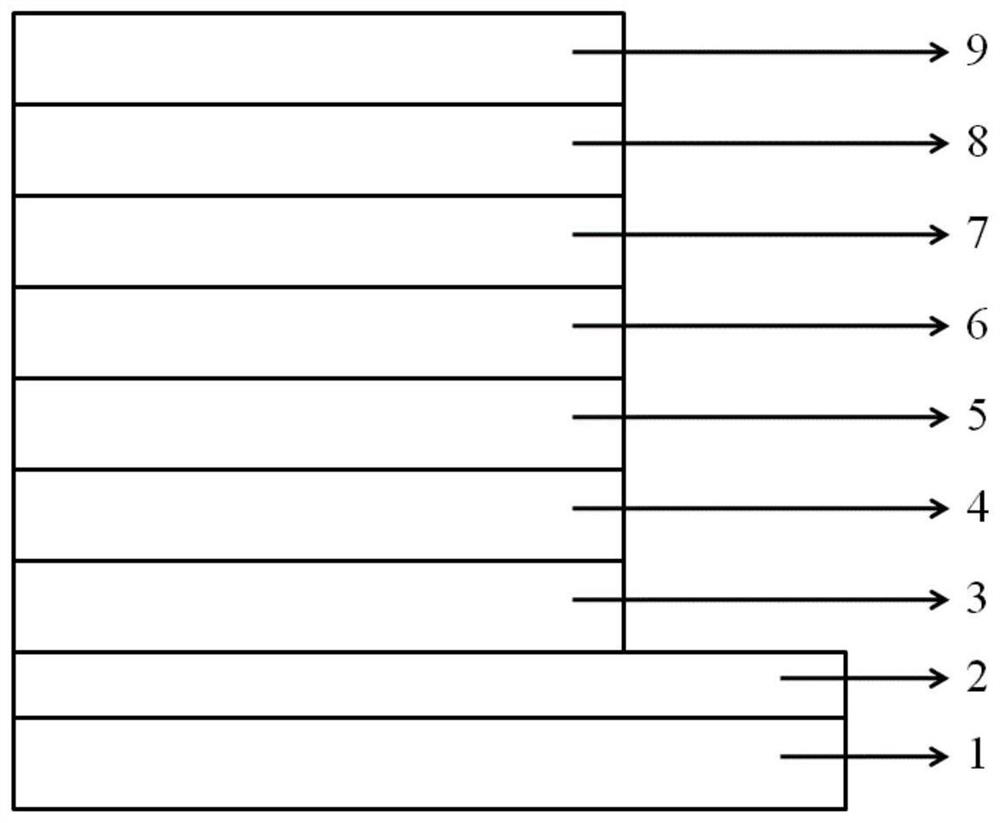
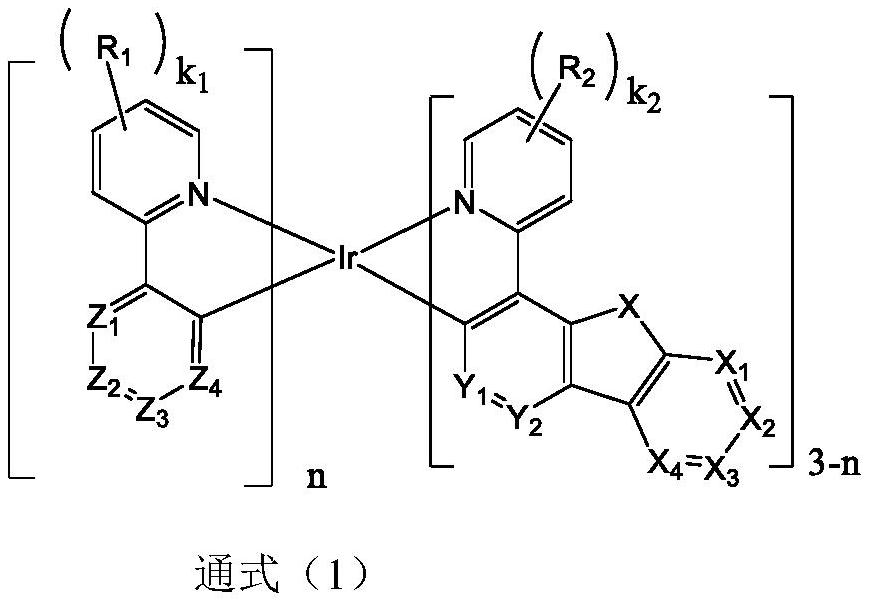
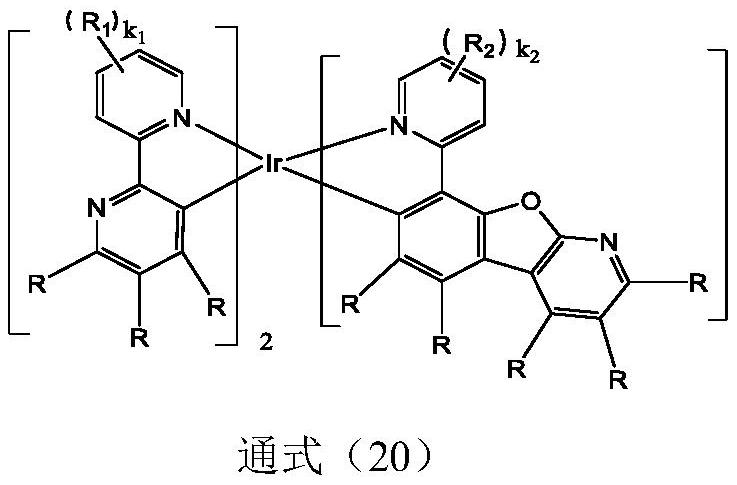
Organic electroluminescent device based on iridium-containing organic complex as electroluminescent material
An electroluminescent device and organic compound technology, applied in the field of organic electroluminescent devices, can solve the problems of reduced device efficiency and lifespan, easy decomposition of luminescent materials, increased device driving voltage, etc. Extraction effect, effect of reducing driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Synthesis Example 1: Synthesis of Compound D-2:
[0089]
[0090] Add 2.0mmol of intermediate A and 5.0mmol of intermediate B-1 into the reaction flask, add 50ml of ethanol, and reflux for 24 hours under nitrogen protection. During the reaction, solids are precipitated. After the reaction, filter, and the obtained solids are passed through the column and washed. The solvent removal ratio is petroleum ether:dichloromethane=3:1, and a yellow-green solid is obtained with a purity of 98.6%.
[0091] Elemental Analysis: Theoretical; C, 58.33; H, 3.31; Ir, 25.23; N, 11.03; O, 2.10. Found; C, 58.29; H, 3.33; Ir, 25.26; N, 11.01; O, 2.11. Proton NMR spectrum: 1H NMR (500MHz, Chloroform-d) δ8.65(ddd,3H),8.56(ddd,4H),8.09(d,1H),7.78(dd,1H),7.67(td,1H), 7.64-7.55(m,5H),7.42(d,1H),7.26-7.11(m,6H),2.53(d,3H).
Embodiment 2
[0092] Synthesis Example 2: Synthesis of Compound D-9:
[0093]
[0094] The preparation method of compound D-9 is the same as that in Synthesis Example 1, except that raw material B-7 is replaced by raw material B-1, and the purity is 98.2%.
[0095] Elemental Analysis: Theoretical; C, 62.03; H, 3.67; Ir, 22.56; N, 9.86; O, 1.88. Found; C, 62.01; H, 3.68; Ir, 22.57; N, 9.87; Proton NMR spectrum: 1H NMR (500MHz, Chloroform-d) δ8.57(dt, 4H), 8.51(d, 1H), 8.09(d, 1H), 7.93 (dd, 2H), 7.64–7.55(m, 5H ),7.47–7.39(m,3H),7.39–7.32(m,1H),7.32–7.26(m,3H),7.25–7.11(m,5H),2.53(d,3H),2.47(d,3H ).
Embodiment 3
[0096] Synthesis Example 3: Synthesis of Compound D-10:
[0097]
[0098] The preparation method of compound D-10 is the same as in Synthesis Example 1, except that intermediate A-5 is used to replace intermediate A, and intermediate B-8 is used to replace intermediate B-1, with a purity of 98.5%.
[0099] Elemental Analysis: Theoretical; C, 65.97; H, 4.31; Ir, 19.55; N, 8.55; O, 1.63. Found; C, 65.94; H, 4.30; Ir, 19.57; N, 8.57; O, 1.62. Proton NMR: 1H NMR (500MHz, Chloroform-d) δ8.97(s,2H),8.64–8.56(m,3H),8.42–8.38(m,2H),7.95(d,1H),7.61–7.54 (m,2H),7.54–7.46(m,6H),7.43(d,1H),7.41–7.29(m,6H), 7.24(dq,1H),7.15(ddq,1H),2.58–2.48(m ,15H),2.39–2.35(m,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com