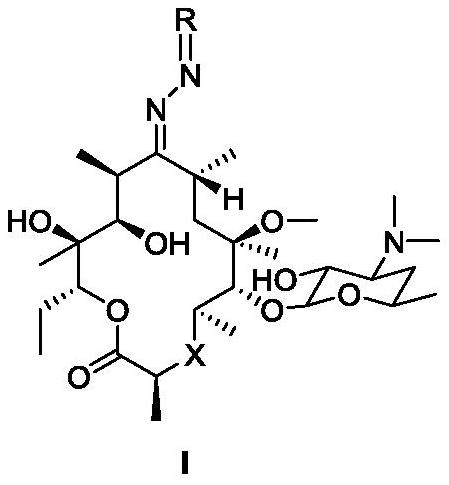
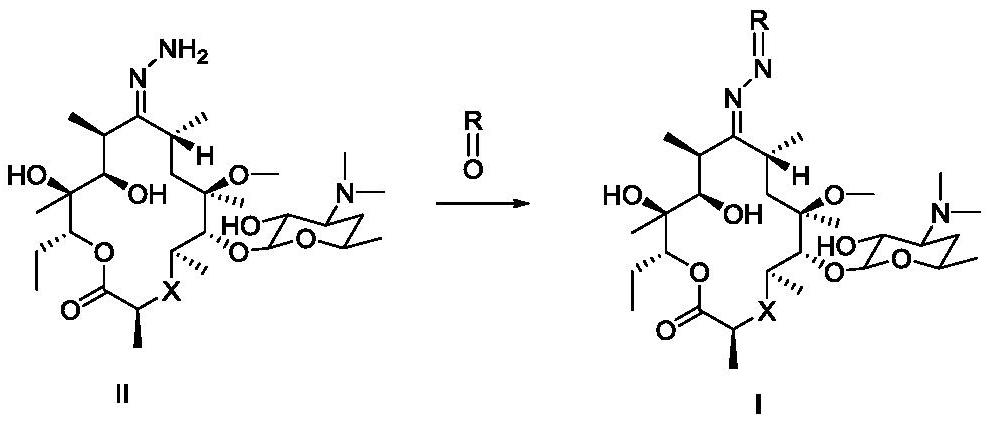
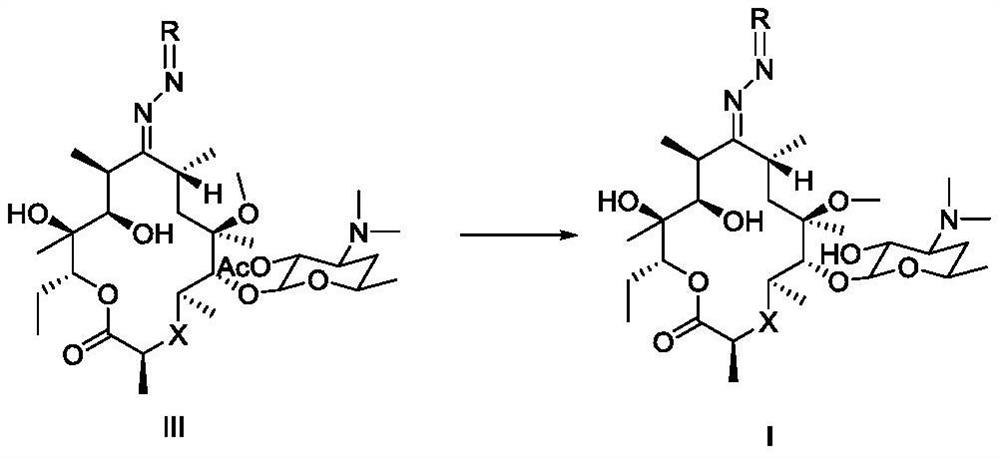
Macrolide compound and synthesis method, pharmaceutical composition and application of compound
A macrolide and compound technology, applied in the field of macrolide compounds, can solve the problems of the lack of structural diversity of antibiotics, the development and spread of drug resistance, etc., and achieve good in vitro synergy effect and good market. Development prospects, effects of alleviating drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19
[0174] Example 1 9-hydrazone clarithromycin (intermediate 2)
[0175] Step 1: Preparation of hydrazine acetate
[0176] Glacial acetic acid (168ml, 2.94mol) was added dropwise into 85% hydrazine hydrate (140ml, 2.45mol) under stirring in an ice bath, during which mechanical stirring was used and the reaction temperature was controlled not to exceed 10°C. Remove the ice bath after the addition of glacial acetic acid is completed, turn to room temperature and stir for 40 minutes, during which solids will precipitate out, filter with suction, wash the solids with 50ml of ethanol, then continue to add the solids to 100ml of absolute ethanol and stir for 10 minutes, filter with suction, and Repeat the above operation. The solid obtained by suction filtration was put into a 500ml eggplant-shaped bottle and vacuum-dried to obtain 198g of white crystals, with a yield of 87.90%.
[0177] Step 2: Preparation of pyridinium trifluoroacetate
[0178] Pyridine (16ml, 0.2mol) was dissolve...
Embodiment 2
[0182] Example 2 3-hydroxyl 9-hydrazone clarithromycin (intermediate 3)
[0183]
[0184] Add 9-hydrazone clarithromycin (10 g, 13.1 mmol) and 50 ml of 1 N aqueous HCl into a 100 ml round bottom flask, and stir at room temperature for 4 h. Add 30ml of dichloromethane, adjust the pH to 9-10 with 3 equivalents of NaOH solution, separate the layers, extract the aqueous layer twice with 10ml of DCM, wash the organic phase with water and saturated brine successively, spin dry, and dry in vacuo to obtain a white Solid 8.61g, yield 86%.
Embodiment 3
[0185] Example 3 2'-O-acetyl-3-hydroxyl-9-isopropylidene hydrazone clarithromycin (intermediate 4)
[0186]
[0187]Dissolve 8.61 g of 3-hydroxy 9-hydrazone clarithromycin in 50 ml of acetone, stir at room temperature for 4 h, monitor the reaction by TLC, add 0.17 ml of acetic anhydride after completion, stir at room temperature for 3 h, spin dry and add 40 ml of dichloromethane, and then wash the organic phase with distilled water , washed with saturated brine, spin-dried, and vacuum-dried to obtain 8.55 g of a white solid with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com