Preparation method of 5-(alpha-halogenated butyryl)-8-hydroxyquinoline-2-ketone
A technology of halobutyryl and halobutyryloxy, applied in organic chemistry and other directions, can solve problems such as unfavorable industrial production, excessive waste brine, etc., to save preparation time, controllable reaction conditions, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
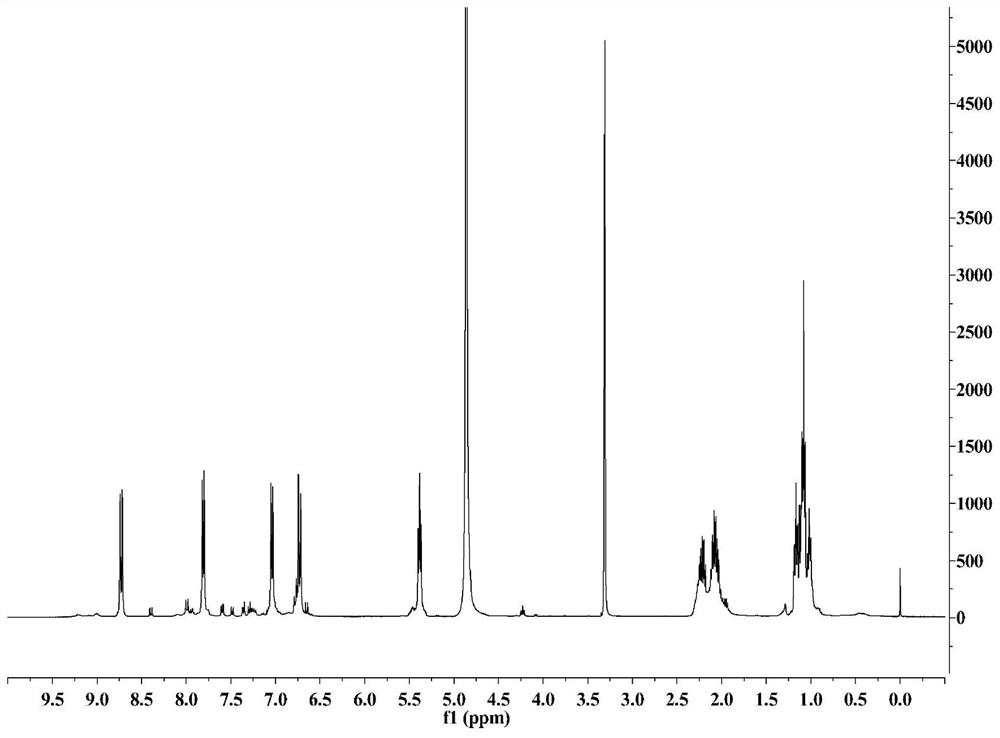
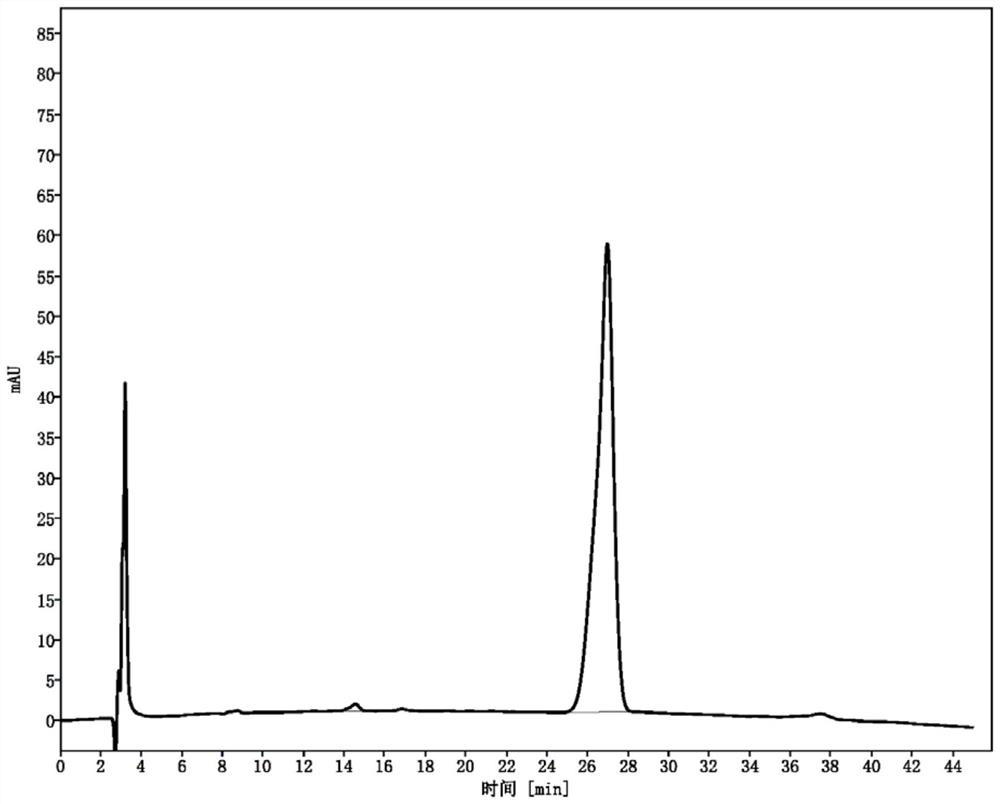
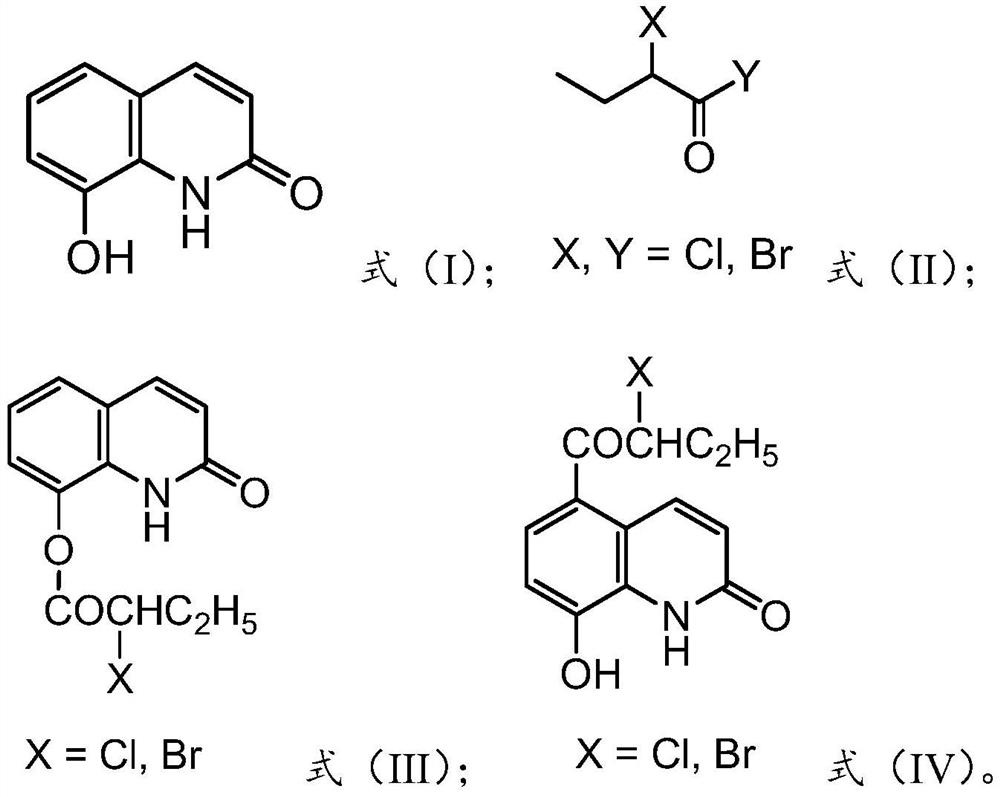
[0034] The present invention provides a kind of preparation method of 5-(alpha-halobutyryl)-8-hydroxyquinolin-2-one, comprising the following steps:
[0035] Step 1: In the presence of an acid-binding agent, the 8-hydroxyquinolin-2-one shown in formula (I) is acylated with the acylating agent shown in formula (II) to obtain the formula ( Ⅲ) 8-(α-halobutyryloxy)-quinolin-2-one shown in;
[0036] Step 2: Rearrange 8-(α-halobutyryloxy)-quinolin-2-one in methanesulfonic acid to obtain 5-(α-halobutyrate as shown in formula (IV) Acyl)-8-hydroxyquinolin-2-one;
[0037]
[0038] Preferably, the molar ratio of 8-hydroxyquinolin-2-one to acylating agent is 1:(1-1.5).
[0039] Preferably, the molar ratio of 8-(α-halobutyryloxy)-quinolin-2-one to methanesulfonic acid is 1:(5-15).
[0040] Preferably, the acylating agent includes one or more of 2-chlorobutyryl chloride, 2-bromobutyryl chloride, 2-chlorobutyryl bromide and 2-bromobutyryl bromide.
[0041] Preferably, the acid-binding...
Embodiment 1
[0059] Example 1: 8-(α-chlorobutyryloxy)-quinolin-2-one
[0060] 16.1 g (0.10 mol) of 8-hydroxyquinolin-2-one was put into 150 ml of anhydrous dichloromethane, 34.6 g (0.30 mol) of diisopropylethylamine was added, and 15.5 g of 2-chlorobutyryl chloride was slowly added dropwise at room temperature. g (0.11mol), after the addition, keep stirring at 40°C for 4h, add 200ml of water and stir for 0.5h, filter the mixture directly, wash the filter cake with an appropriate amount of water, and dry the filter cake under vacuum at 50°C to obtain 24.7g of white powder with a yield of 93.0 %.
Embodiment 2
[0061] Example 2: 8-(α-bromobutyryloxy)-quinolin-2-one
[0062] 16.1 g (0.10 mol) of 8-hydroxyquinolin-2-one was dropped into 150 ml of anhydrous dichloromethane, 17.7 g (0.30 mol) of triethylamine was added, and 25.3 g of 2-bromobutyryl bromide was slowly added dropwise at room temperature ( 0.11mol), after the addition, keep stirring at 40°C for 4h, add 200ml of water and stir for 0.5h, filter the mixture directly, wash the filter cake with an appropriate amount of water, and vacuum-dry the filter cake at 50°C to obtain 25.5g of white powder with a yield of 82.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com