Pharmaceutical preparation for avoiding or reducing generation of N-nitrosamine genotoxic substances
A technology of genotoxicity and nitrosamines, applied in the field of medicine, can solve problems such as genotoxic impurities that cannot be solved, achieve the effect of reducing the production of genotoxic substances and improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Ranitidine 300 mg bilayer tablet in a 50:50 immediate release:sustained release ratio.
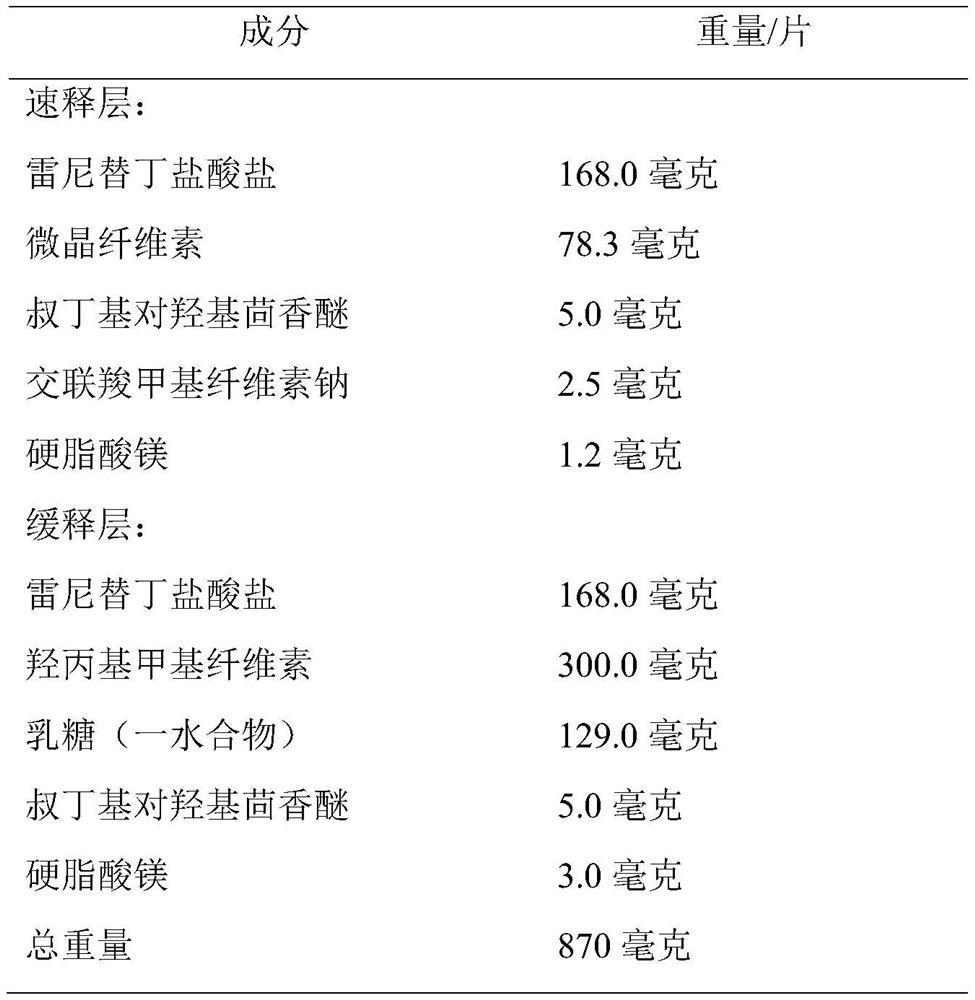
[0041] Group distribution ratio:
[0042]
[0043] Note: The "300 mg" tablet in the present invention refers to the weight of ranitidine free base in the tablet; the mass ratio of tert-butyl-p-hydroxyanisole to ranitidine free base is 1:30.
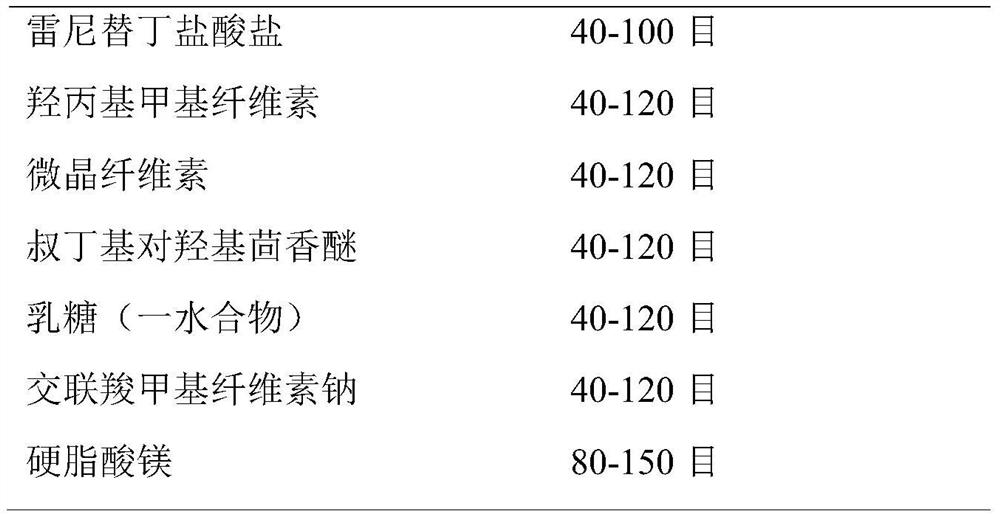
[0044] (1) Particle size control of raw and auxiliary materials:
[0045] The particle size of raw and auxiliary materials is controlled by standard sieve:
[0046]
[0047]
[0048] (2) Mixing of slow-release layer materials:
[0049] Add ranitidine hydrochloride, lactose (monohydrate), hydroxypropyl methylcellulose, tert-butyl-p-hydroxyanisole to a double wall blender and blend for 30 minutes, then add stearin to the above mixture magnesium acid, and the mixture was stirred again for 1 minute.
[0050] Immediate release layer material mixing
[0051] Add ranitidine hydrochloride, microcrystalline cellulose, croscarmellose sodium...
Embodiment 2
[0056] 75mg ranitidine calcium carbonate tablets:
[0057]
[0058] Note: The "75 mg" tablet in the present invention refers to the weight of ranitidine free base in the tablet; the mass ratio of ascorbyl palmitate to ranitidine free base is 15:1.
[0059] Tablet Compression: Combine, mix and compress the appropriate amounts of the ingredients above into small pieces. These small pieces are ground to form granules which can pass through a 14-16 mesh screen and can be compressed into tablets using a suitable compression die.
[0060] No antioxidant was added, and the bilayer tablet prepared by the same preparation method as in Example 2 was Comparative Example 2.
Embodiment 3
[0062] 51 grams of sodium carboxymethylcellulose (Blanose 7HF) was mixed with 500 grams of metformin hydrochloride and granulated with 95% ethanol in an asteroid mixer. The wet granules were passed through a 2 mm mesh screen and then dried in an oven at 55°C for 1 hour. The dried granules (530 grams) were mixed with 344 grams of hydroxypropyl methylcellulose 2208USP (grade 100,000cps), 9.5 grams of hydroxypropyl methylcellulose 2910USP (grade 5cps), 10 grams of ascorbic acid (vitamin C), and 100 grams of micro Crystalline cellulose (mass ratio of ascorbic acid to metformin hydrochloride is 1:50), mixed in a planetary mixer for 10 minutes. Finally, the mixture was mixed with 1% by weight of magnesium stearate and compressed into capsule-like tablets, each tablet containing 500 mg of metformin hydrochloride.
[0063] Without adding antioxidant, the bilayer tablet prepared by the same preparation method as Example 3 is Comparative Example 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com