A kind of synthetic method of imidazolinone derivative
A technology of imidazolinone and synthesis method, which is applied in the field of synthesis of imidazolinone derivatives, can solve the problems of high equipment requirements, cumbersome steps, low yield, etc., and achieve the effect of meeting the synthesis requirements and having great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The first embodiment of the present invention is: a method for synthesizing imidazolidinone derivatives, comprising the following steps:
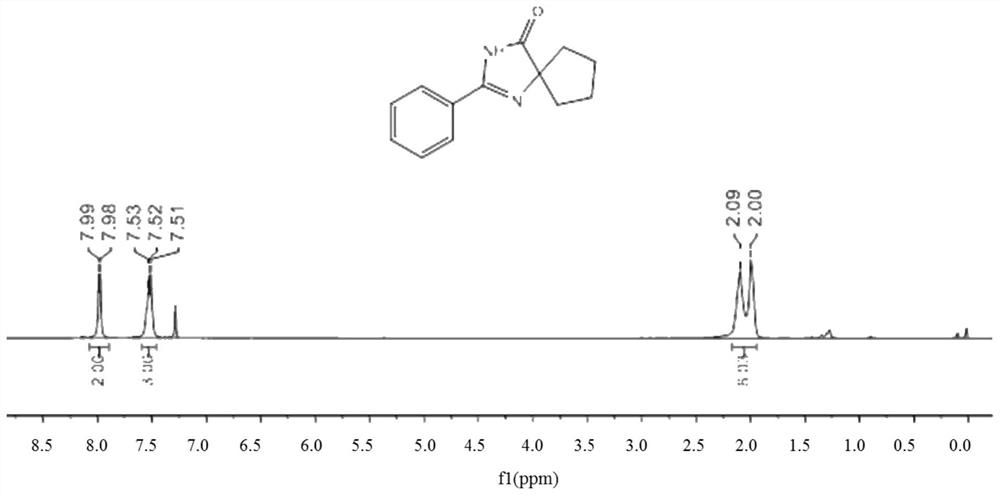
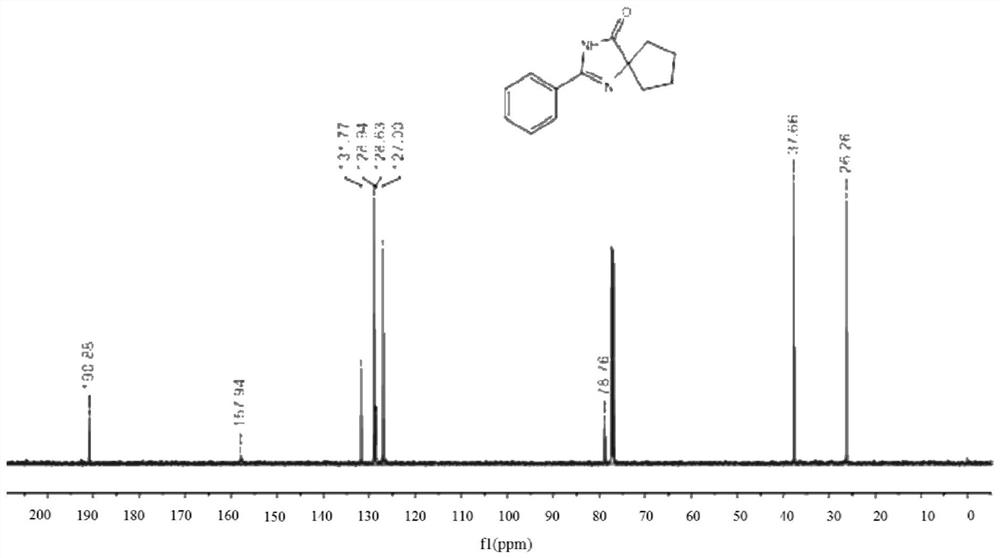
[0069] 0.25 mmol of benzamidine hydrochloride, 0.375 mmol of 1,2-cyclohexanediol, 0.6 mmol of cesium carbonate, 60 mg of activated carbon-supported cobalt metal catalyst (activated carbon mass fraction of 3%) and 1 ml of pyridine, stirred and reacted at 130 ° C under air conditions for 12 hours, stopped heating and stirring, cooled to room temperature, evaporated under reduced pressure to remove the solvent, and then separated and purified by thin-layer chromatography to obtain The target product, the stationary phase of the thin-layer chromatography used is silica gel, the eluent is a mixed solvent of petroleum ether and ethyl acetate (petroleum ether:ethyl acetate=5:1, v / v), and the yield of the obtained product is 89% .
[0070] The hydrogen spectrogram and carbon spectrogram of the product obtained in Example 1 of the present in...
Embodiment 2
[0077] The second embodiment of the present invention is: a method for synthesizing imidazolidinone derivatives, comprising the following steps:
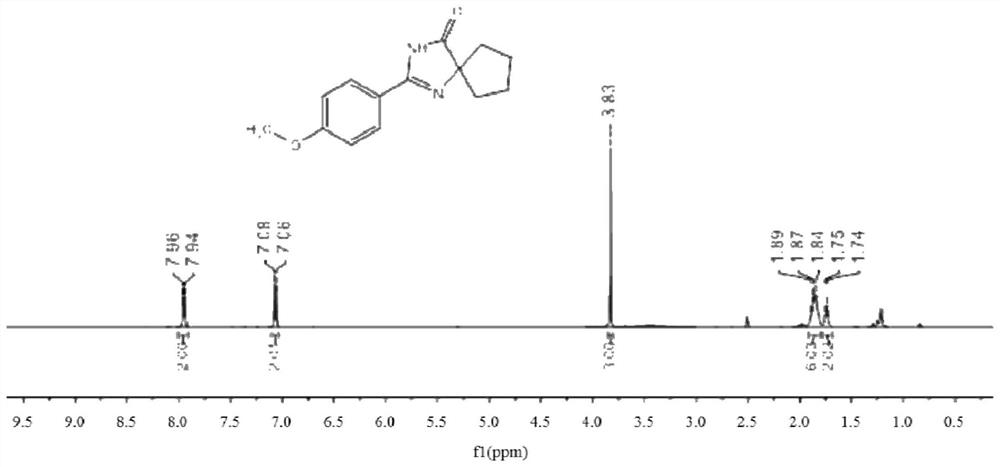
[0078] In a schlenk tube were added 0.25 mmol 4-methoxybenzamidine hydrochloride, 0.45 mmol 1,2 cyclohexanediol, 0.5 mmol potassium hydroxide, 45 mg alumina supported cobalt metal catalyst (trioxide). The mass fraction of aluminum oxide is 3%) and 1 ml of tert-amyl alcohol, and the reaction was stirred at 110 ° C and air for 12 hours, then heating and stirring were stopped, cooled to room temperature, and the solvent was removed by rotary evaporation under reduced pressure, and then passed through a thin layer of Chromatographic separation and purification to obtain the target product, the stationary phase of the thin-layer chromatography used is silica gel, and the eluent is a mixed solvent of petroleum ether and ethyl acetate (petroleum ether:ethyl acetate=5:1, v / v), the obtained The yield of product was 84%.
[0079] The hydroge...
Embodiment 3
[0086] The third embodiment of the present invention is: a method for synthesizing imidazolidinone derivatives, comprising the following steps:
[0087] In a schlenk tube, add 0.25 mmol 4-fluorobenzamidine hydrochloride, 0.5 mmol 1,2 cyclohexanediol, 0.5 mmol sodium methoxide, and 60 mg titanium dioxide-supported nano-cobalt catalyst (the mass fraction of titanium dioxide is 3 %) and 1 ml of methanol, stirred and reacted at 120 ° C under air conditions for 10 hours, stopped heating and stirring, cooled to room temperature, evaporated under reduced pressure to remove the solvent, and then separated and purified by thin-layer chromatography to obtain the target product. The stationary phase of thin layer chromatography is silica gel, the eluent is a mixed solvent of petroleum ether and ethyl acetate (petroleum ether:ethyl acetate=7:1, v / v), and the yield of the obtained product is 83%.
[0088] The hydrogen spectrogram and carbon spectrogram of the product obtained in Example 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com