A kind of C2 substituted 2h-benzothiazole aryl acylation derivative and its synthetic method and application
A technology for the synthesis of benzothiazole aryl acyl derivatives, which is applied in the field of C2 substituted 2H-benzothiazole aryl acylate derivatives and their synthesis, can solve the problems of yield reduction, flammability and explosion of organic peroxide oxidants, The problems such as the increase of reaction by-products have been solved, and the catalytic system is simple, no transition metal catalyst is needed, and the product yield is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
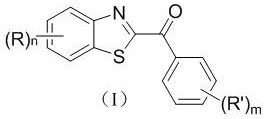
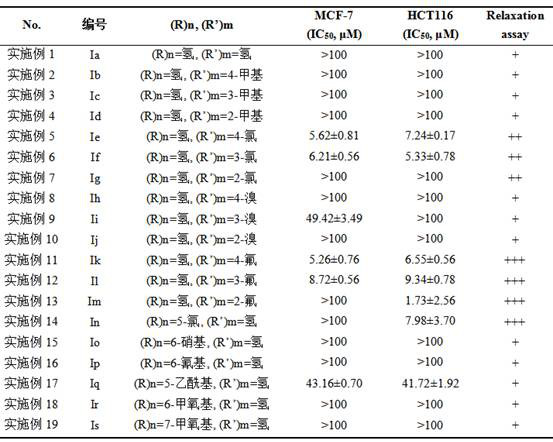
[0024]Example 1 Synthesis of derivative Ia ((R)n=hydrogen, (R')m=hydrogen)
[0025] Weigh 2 H - Benzothiazole (0.3 mmol, 40.6 mg), toluene (7.5 mmol, 691.1 mg), Selectfluor (0.6 mmol, 212.6 mg) and trifluoroacetic acid (0.3 mmol, 34.2 mg) in a 25 mL reaction tube, followed by 2 mL of acetonitrile was placed at 80 °C in an air atmosphere for the reaction, monitored by TLC, the reaction was completed after about 10 h, the reaction solution was extracted with dichloromethane, the organic layer was concentrated to remove the solvent, and the concentrated solution was separated by column chromatography (eluent: volume ratio of 1:0.05 petroleum ether-ethyl acetate mixed solvent) to obtain a yellow solid, namely derivative Ia. Yield 85%.
[0026] of the compound 1 H NMR and 13 C NMR analysis data are as follows,
[0027] 1 H NMR (500 MHz, CDCl 3 ) δ 8.58 (dd, J = 8.5, 1.0 Hz, 2H), 8.28 – 8.25(m, 1H), 8.07 – 8.03 (m, 1H), 7.72 – 7.67 (m, 1H), 7.63 – 7.56 (m, 4H); 13 CNMR (...
Embodiment 2
[0031] Example 2 Synthesis of derivative Ib ((R)n=hydrogen, (R')m=4-methyl)
[0032] Weigh 2 H - benzothiazole (0.3 mmol, 40.6 mg), p-xylene (8.0 mmol, 848.6 mg), Selectfluor (0.3 mmol, 106.3 mg) and trifluoroacetic acid (0.45 mmol, 51.3 mg) in a 25 mL reaction tube, and then 2 mL of acetonitrile was added, and the reaction was performed at 70 °C in an air atmosphere. TLC monitoring was performed. After about 8 h, the reaction was completed. The reaction solution was extracted with dichloromethane, the organic layer was concentrated to remove the solvent, and the concentrated solution was separated by column chromatography (eluting). The solvent is petroleum ether-ethyl acetate mixed solvent with a volume ratio of 1:0.05) to obtain a yellow solid, namely derivative Ib. Yield 96%.
[0033] of the compound 1 H NMR and 13 C NMR analysis data are as follows,
[0034] 1 H NMR (500 MHz, CDCl 3 ) δ 8.50 (d, J = 8.5 Hz, 2H), 8.28 – 8.24 (m, 1H), 8.05 – 8.02 (m, 1H), 7.62 – ...
Embodiment 3
[0036] Example 3 Synthesis of derivative Ic ((R)n=hydrogen, (R')m=3-methyl)
[0037] Weigh 2 H - benzothiazole (0.3 mmol, 40.6 mg), m-xylene (8.0 mmol, 848.6 mg), Selectfluor (0.3 mmol, 106.3 mg) and trifluoroacetic acid (0.45 mmol, 51.3 mg) in a 25 mL reaction tube, and then 2 mL of acetonitrile was added, and the reaction was performed at 70 °C in an air atmosphere. TLC monitoring was performed. After about 8 h, the reaction was completed. The reaction solution was extracted with dichloromethane, the organic layer was concentrated to remove the solvent, and the concentrated solution was separated by column chromatography (eluting). The solvent is petroleum ether-ethyl acetate mixed solvent with a volume ratio of 1:0.05) to obtain a yellow solid, namely derivative Ic. The yield is 88%.
[0038] of the compound 1 H NMR and 13 C NMR analysis data are as follows,
[0039] 1 H NMR (500 MHz, CDCl 3 ) δ 8.40 (d, J = 7.5 Hz, 1H), 8.30 (s, 1H), 8.29– 8.26 (m, 1H), 8.05 – 8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com