Compound as well as preparation method and application thereof
A compound and heterocyclic compound technology, applied in the field of medicine, can solve problems such as unsatisfactory curative effect and anticancer drugs that need to be deepened, and achieve the effect of increasing yield, improving yield and reaction efficiency, and inhibiting cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
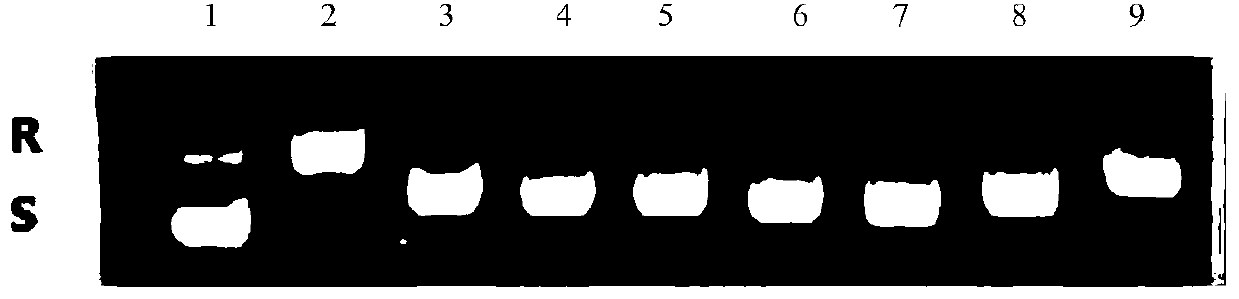
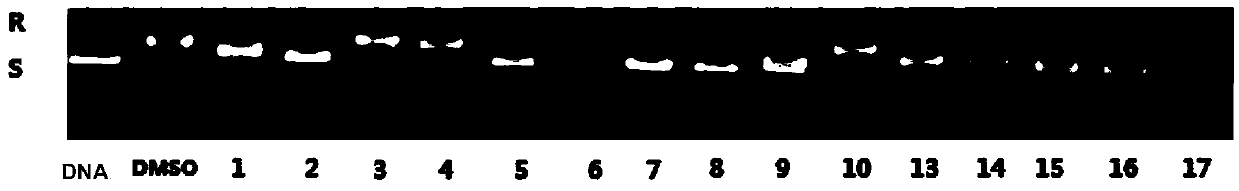
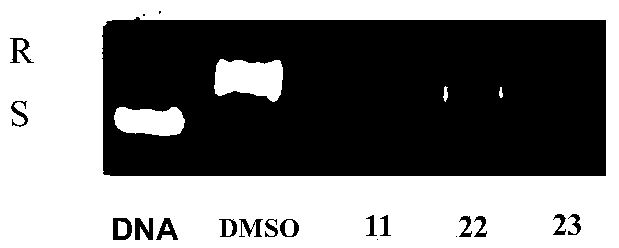
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of 9-[(2-benzimidazolyl)-methyl]-2-methoxy-acridin-9-amine
[0060] 1. Preparation of 2-(4-methoxyphenylamino)benzoic acid
[0061] Add 2-chlorobenzoic acid (1.00g, 5.23mmol), 4-methoxyaniline (0.86g, 6.99mmol), potassium carbonate (2.00g, 14.49mmol) in dimethylformamide (DMF, 50.00ml) and copper powder (0.20g, 3.15mmol), then stirred overnight at 130°C, then added to 200ml of water after the resulting reaction mixture was cooled, the resulting mixture was adjusted to a pH value of about 3 with hydrochloric acid, suction filtered and The obtained precipitate was dried to obtain an off-white solid, namely 2-(4-methoxyphenylamino)benzoic acid, and the product was directly carried out to the next reaction without further purification.
[0062] 2. Preparation of 9-chloro-2-methoxyacridine
[0063] The 2-(4-methoxyphenylamino)benzoic acid (2.00g, 7.19mmol) obtained in step 1 was added to anhydrous phosphorus oxychloride (10.00ml), and refluxed at 110...
Embodiment 2
[0070] Embodiment 2: Preparation of 9-[(2-benzimidazolyl)-methyl]-acridine-9 amine
[0071] 9-chloroacridine (1 mmol) and 2-aminomethylbenzimidazole (1.2 mmol) were added to 2 g of phenol, stirred at 120° C. for 2 hours, and then 100 ml of ethyl acetate was added to the resulting reaction solution to produce A large amount of precipitation was filtered, and the resulting precipitate was washed with ether to obtain 9-[(2-benzimidazolyl)-methyl]-acridine-9amine (ie, the compound shown in formula 1).
[0072] Yield 32%; melting point 262°C; high-resolution mass spectrum (ESI): calculated value [M+H]+325.1453; experimental value: 325.1447.
[0073] The confirmed data of the compound structure are:
[0074] 1 H NMR(400MHz,DMSO)δ8.66(d,J=8.1Hz,2H),7.98(d,J=13.8Hz,4H),7.54(d,J=16.2Hz,4H),7.19(dd,J =6.0,3.0Hz,2H),5.54(s,2H).
Embodiment 3
[0075] Example 3: Preparation of 9-[(2-benzoimidazolyl)-methyl]-6-chloro-2-methoxy-acridin-9-amine
[0076] 6,9-Dichloro-2-methoxy-acridine (1mmol) and 2-aminomethylbenzimidazole (1.2mmol) were added to 2g of phenol, stirred at 120°C for 2 hours, and then to the obtained 100ml of ethyl acetate was added to the reaction solution to produce a large amount of precipitate, which was filtered by suction and washed with ether to obtain 9-[(2-benzoimidazolyl)-methyl]-6-chloro-2-methoxy- Acridin-9-amine (i.e. the compound shown in formula 3).
[0077] Yield 22%; melting point 237°C; high-resolution mass spectrum (ESI): calculated value [M+H]+389.1169; experimental value: 389.1163.
[0078] The confirmed data of the compound structure are:
[0079] 1 H NMR(400MHz,DMSO)δ8.66(d,J=8.1Hz,2H),7.98(d,J=13.8Hz,4H),7.54(d,J=16.2Hz,4H),7.19(dd,J =6.0,3.0Hz,2H),5.54(s,2H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com