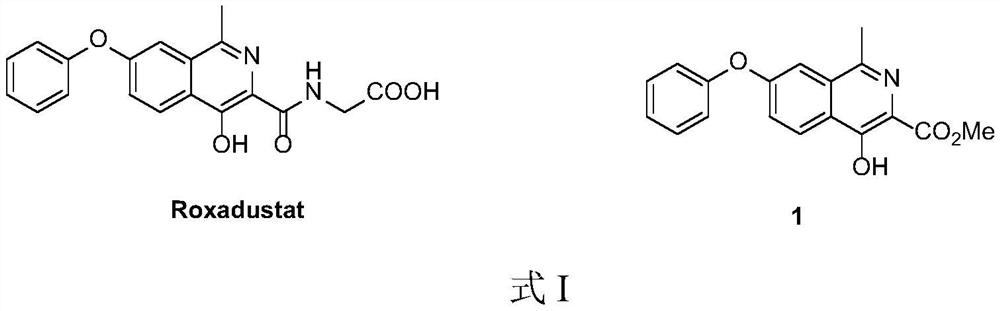
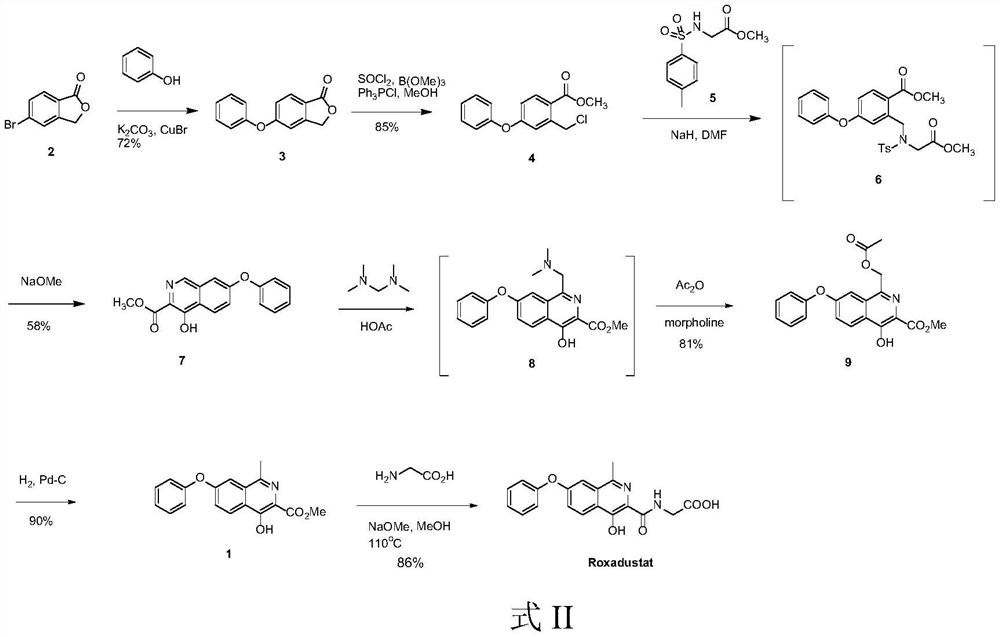
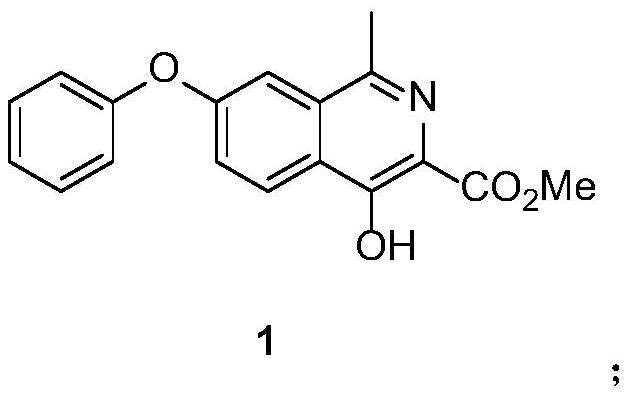
Preparation method of roxadustat intermediate
A technology of roxadustat and an intermediate, which is applied in the fields of organic synthesis and the preparation of raw materials, and can solve the problems of low production efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation process of m-phenoxyacetophenone (compound 10 in formula III, hereinafter referred to as compound 10), is as follows:
[0033] Take a 1L three-necked flask, add DMF 300mL, potassium carbonate (62g, 0.45mol) in turn, stir, add 3-bromoacetophenone (60g, 0.3mol), phenol (31g, 0.33mol), cuprous bromide (14.3g , 0.1mol), under nitrogen protection, heated to 80-90°C for 6 hours, followed by TLC to complete the reaction, cooled to room temperature, poured the reactant into 1kg of water, stirred, added 800mL of ethyl acetate, stood still to separate the liquid, and washed the organic phase with water (500mL×3), the organic phase was concentrated and dried under reduced pressure to obtain a yellow oil, which was distilled under reduced pressure, and fractions (1mmHg) at 145-150°C were collected to obtain compound 10 (52g, 82%) as a colorless liquid.
[0034] The prepared compound 10 1 The nuclear magnetic resonance spectra of H NMR and MS (ESI) are as follows:
...
Embodiment 2
[0040] A preparation process of m-phenoxyacetophenone (compound 10 in formula III, hereinafter referred to as compound 10), is as follows:
[0041]Take a 1L three-necked flask, add toluene 600mL, potassium hydroxide (28g, 0.5mol), stir, add 3-bromoacetophenone (50g, 0.25mol), phenol (28g, 0.30mol), copper acetate (20g, 0.1 mol), nitrogen protection, reflux reaction for 10h, TLC traced the end of the reaction, cooled to room temperature, filtered off the insoluble matter, washed the organic phase (500mL×2), concentrated and dried the organic phase under reduced pressure to obtain a yellow oil, and purified by column chromatography to obtain the compound 10 (38 g, 73%).
[0042] Its spectrogram detection result is with embodiment 1.
Embodiment 3
[0044] A preparation process of methyl 2-nitro-3,3-dimethoxypropionate (compound 11 in formula III, hereinafter referred to as compound 11), specifically as follows:
[0045] Add methyl nitroacetate (60 g, 0.5 mol) and trimethyl orthoformate (70 g, 0.66 mol) into acetic anhydride (100 g), and react at 80° C. for 12 h. Concentration under reduced pressure gave a yellow oil, which was distilled under reduced pressure, and the 110-115°C fraction (1 mmHg) was collected to obtain light yellow liquid compound 11 (39 g, 84%).
[0046] The prepared compound 11 1 The nuclear magnetic resonance spectrum of H NMR is as follows:
[0047] 1 H NMR (400MHz, CDCl 3 )δ5.79-5.66(m,1H),5.10-5.17(m,1H),3.66(s,3H),3.41(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com