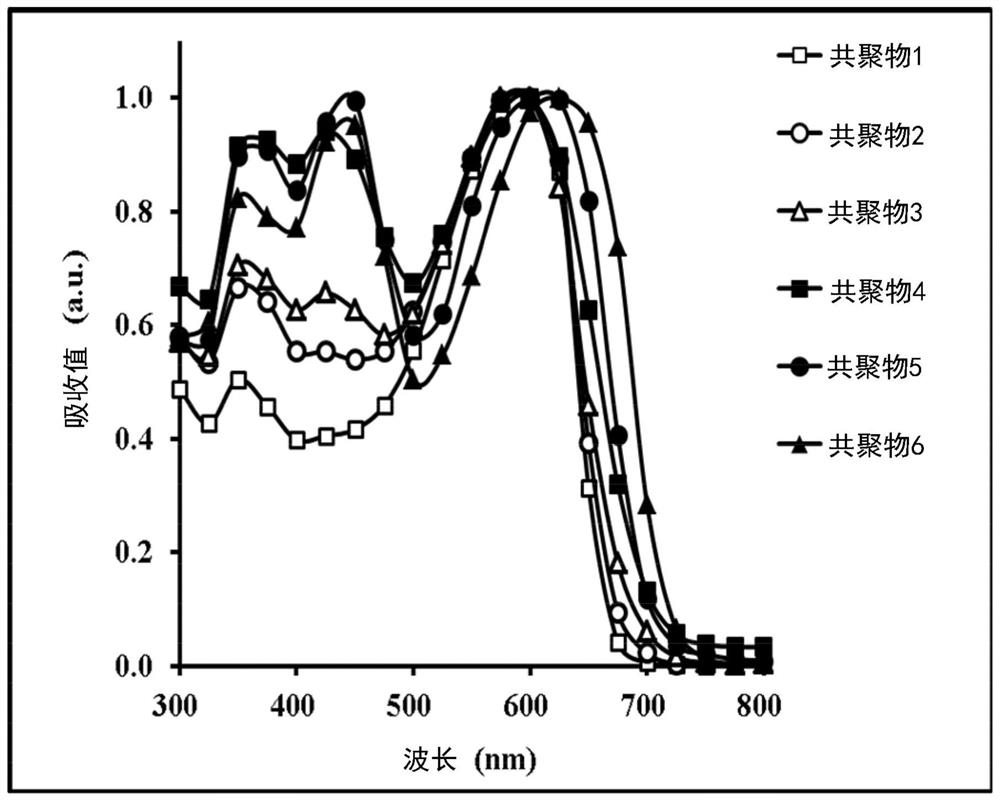
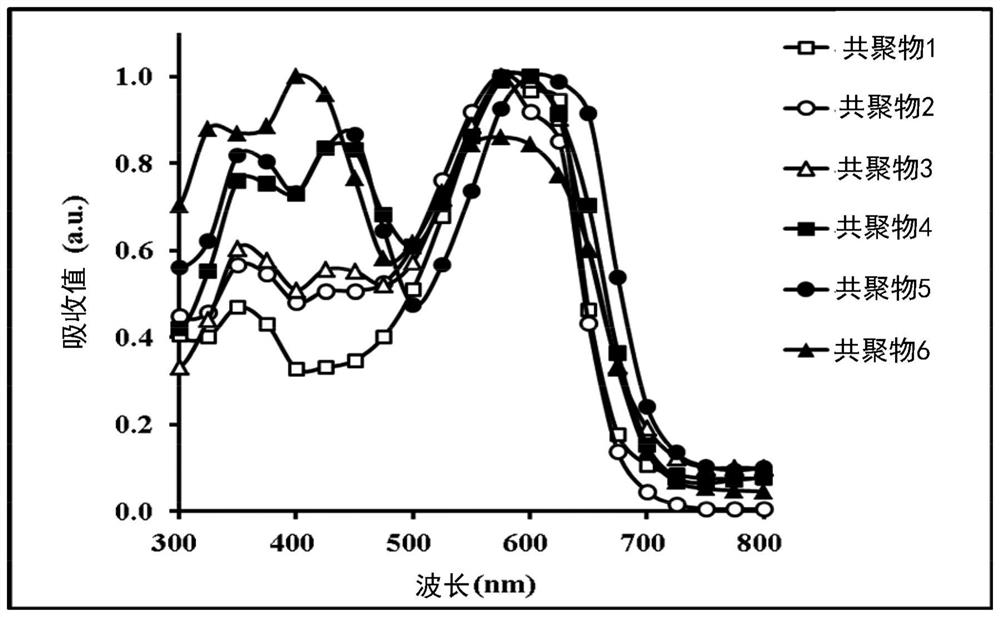
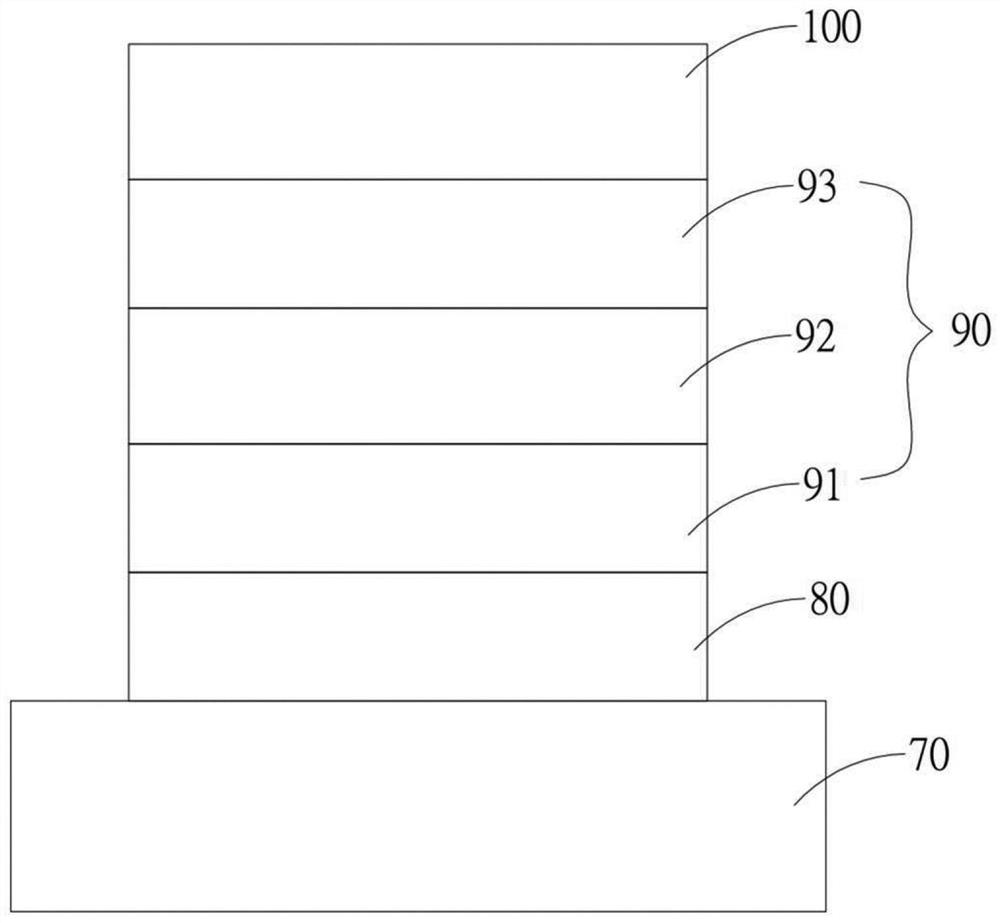
Copolymer and organic photovoltaic element
A kind of technology of copolymer and copolymerization ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0079] Preparation of compound 8
[0080] Compound 8 was prepared according to the following method.
[0081]
[0082] The preparation method of compound 2:
[0083] Under nitrogen, compound 1 (10mmol) and sodium borohydride (NaBH 4 ) (25mmol) was charged into a 250mL reaction flask, and then 100mL of absolute ethanol (EtOH) was added, and heated to 78°C and stirred for 1 hour. Next, after adding water and extracting with dichloromethane and drying over anhydrous magnesium sulfate, the solid was removed by filtration. Finally, the filtrate was concentrated to remove the solvent to obtain compound 2 as a dark brown solid.
[0084]
[0085] The preparation method of compound 4:
[0086] Under nitrogen, compound 3 (12 mmol) was first charged into a 250 mL reaction flask, then 150 mL of anhydrous tetrahydrofuran (THF) was added and the temperature was lowered to 0°C. Next, first add 2.5M n-butyllithium (n-BuLi) in n-hexane solution (12mmol) dropwise and maintain 0°C for...
preparation example 2
[0100] Preparation of compound 13
[0101] Compound 13 was prepared according to the following method.
[0102]
[0103] The preparation method of compound 11:
[0104] Under nitrogen, first feed compound 9 (1 mmol) into a 100 mL reaction flask, then add 15 mL of anhydrous toluene (PhMe) and 0.3 mL of anhydrous dimethylamide (DMF), and then add 1.5 mL of Oxalyl Chloride (COCl) 2 , and stirred at 66 °C for 2 hours. Next, remove all the solvent first, then add aluminum trichloride (AlCl 3 ) (1.5mmol), and then added 20mL of anhydrous dichloromethane (DCM). Next, compound 10 (1 mmol) was added dropwise and after stirring for 1 hour, the reaction was poured into ice. Finally, extraction was performed three times with dichloromethane (DCM). The organic layer was dehydrated by adding magnesium sulfate, concentrated, and then recrystallized with toluene and methanol to obtain compound 11 as a pale yellow solid.
[0105]
[0106] The preparation method of compound 12:
...
Embodiment 1
[0112] Preparation of Copolymer 1
[0113] Copolymer 1 contains repeating units as shown below.
[0114]
[0115] Copolymer 1 was prepared according to the following method.
[0116]
[0117] The preparation method of copolymer 1:
[0118] Under nitrogen, compound 14 (0.50 mmol), compound 13 (0.50 mmol), tris (2-furyl) phosphine [(o-toly) 3 P] (0.08mol) and three (dibenzylideneacetone) dipalladium [Pd 2 (dba) 3 ] (0.02mol) was charged in a 100mL reaction flask. Next, 35 mL of anhydrous chlorobenzene (PhCl) was added, followed by stirring at 130° C. for 4 hours. After the reaction was cooled to room temperature (about 25° C.), the contents of the reaction flask were poured into methanol to precipitate a solid. The precipitate was collected by filtration, and the solid was subjected to Soxhlet extraction with methanol, acetone and chloroform sequentially. Finally, the chloroform residue was poured into methanol for reprecipitation, and the precipitate was collected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com