Heterogeneous hindered Lewis pair catalyst as well as preparation method and application thereof
A Lewis acid-base pair and catalyst technology, applied in the chemical field, can solve the problems of lack of heterogeneous FLPs catalyst reports, and achieve good tolerance, easy separation, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
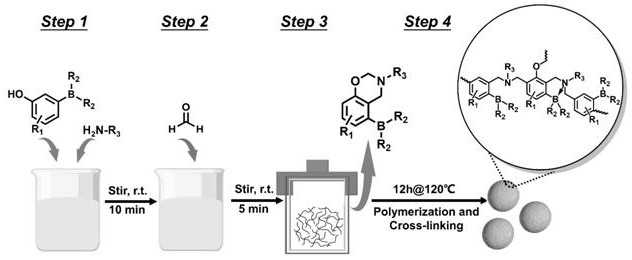
[0030] A hindered Lewis acid-base pair catalyst, prepared from 3-hydroxyphenylboronic acid and ammonia water, the molar ratio of 3-hydroxyphenylboronic acid and ammonia water is 1:6, and the volume ratio of deionized water and absolute ethanol is 3:1, and the reaction The temperature is 120°C, and the synthesis method is as follows: In a 50 mL round bottom flask, dissolve 3-hydroxyphenylboronic acid (1.44 mmol) and ammonia water (8.6 mmol) in 24 ml of deionized water and 8 ml of absolute ethanol, and stir at room temperature for 10 min . Formaldehyde (2.88 mmol) was added and stirred at room temperature for 5 min. The above mixture was transferred to a hydrothermal kettle and placed at 120°C for 12h. Centrifuge, wash, and dry at 100°C for 9 hours. Grind the dried catalyst sufficiently to obtain a hindered Lewis acid-base pair catalyst, which is denoted as BNP-1. The reaction principle is as figure 2 As shown, hydroxyphenylboronic acid monomers, formaldehyde and amines fir...
Embodiment 2
[0033] A hindered Lewis acid-base pair catalyst, prepared from 2-hydroxyphenylboronic acid and ammonia water, the molar ratio of 2-hydroxyphenylboronic acid and ammonia water is 1:6, and the volume ratio of deionized water and absolute ethanol is 3:1, and the reaction The temperature is 120°C, and the synthesis method is as follows:
[0034] In a 50 mL round bottom flask, 2-hydroxyphenylboronic acid (1.44 mmol) and ammonia water (8.6 mmol) were dissolved in 24 ml deionized water and 8 ml absolute ethanol, and stirred at room temperature for 10 min. Formaldehyde (2.88 mmol) was added and stirred at room temperature for 5 min. The above mixture was transferred to a hydrothermal kettle and placed at 120°C for 12h. Centrifuge, wash, and dry at 100°C for 9 hours. The dried catalyst is thoroughly ground to obtain catalyst BNP-2.
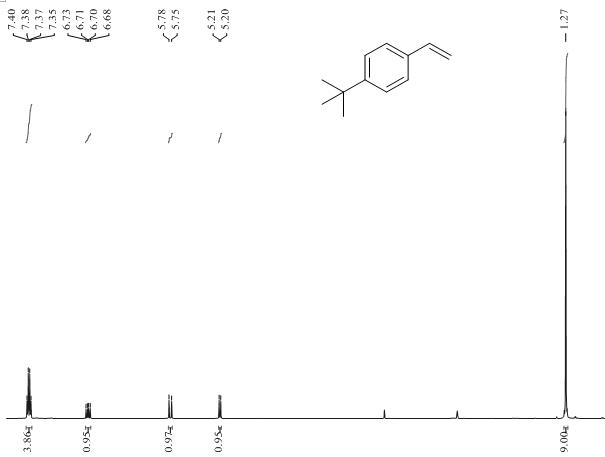
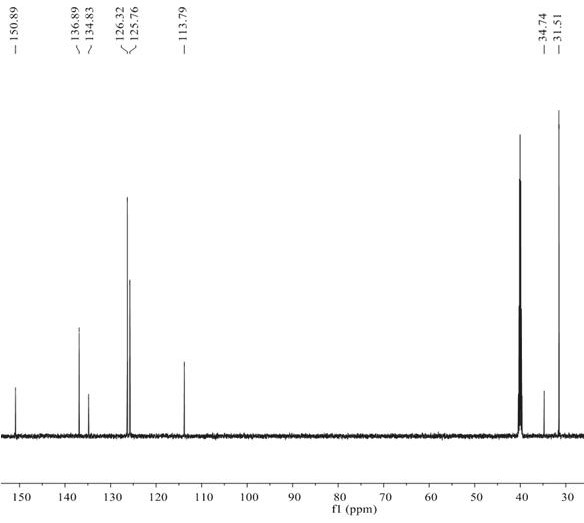
[0035] The method of using BNP-2 for the selective hydrogenation of alkynes to prepare alkenes is as follows: In an autoclave, add p-tert-butylphenylac...
Embodiment 3
[0037] A hindered Lewis acid-base pair catalyst, prepared by 4-hydroxyphenylboronic acid and ammonia water, the molar ratio of 4-hydroxyphenylboronic acid and ammonia water is 1:6, and the volume ratio of deionized water and absolute ethanol is 3:1, and the reaction The temperature is 120°C, and the synthesis method is as follows: In a 50 mL round bottom flask, dissolve 4-hydroxyphenylboronic acid (1.44 mmol) and ammonia water (8.6 mmol) in 24 ml deionized water and 8 ml absolute ethanol, and stir at room temperature for 10 min . Formaldehyde (2.88 mmol) was added and stirred at room temperature for 5 min. The above mixture was transferred to a hydrothermal kettle and placed at 120°C for 12h. Centrifuge, wash, and dry at 100°C for 9 hours. The dried catalyst is thoroughly ground to obtain catalyst BNP-3. It should be pointed out that when 4-hydroxyphenylboronic acid is used as a monomer, the extended carbon chain of the polymer is at the two ortho positions of the hydroxyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com