Oligothiophene functionalized ortho-carborane derivatives, and synthesis method and optical limiting application thereof
An oligomerized thiophene and a synthesis method technology, which can be applied in chemical instruments and methods, compounds containing elements of Group 3/13 of the periodic table, optics, etc., and can solve problems such as limiting the practical application of materials and affecting the stability of devices or sensors. , to enrich assembly behavior, enhance solubility and processability, and facilitate charge transfer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1. Synthesis of Compound 1
[0056] Under the protection of nitrogen, weigh 0.654g (2.0mmol) 5-bromo-2,2':5',2"-tripolythiophene (3T-Br), 46.2mg (0.04mmol) tetrakis (triphenylphosphine) Palladium, 7.6mg (0.04mmol) cuprous iodide and 0.236g (2.4mmol) trimethylsilyl acetylene were placed in a pressure-resistant tube, and 20mL triethylamine and 40mL tetrahydrofuran were added successively to the pressure-resistant tube, and heated to 60°C, stirred and reacted for 12 hours, cooled to room temperature, spin-dried, and carried out column chromatography with a mixture of ethyl acetate and petroleum ether with a volume ratio of 1:20 as the eluent and dried to obtain 0.42 g of bright yellow solid compound 1, Productive rate is about 62%.Its reaction equation is as follows:
[0057]
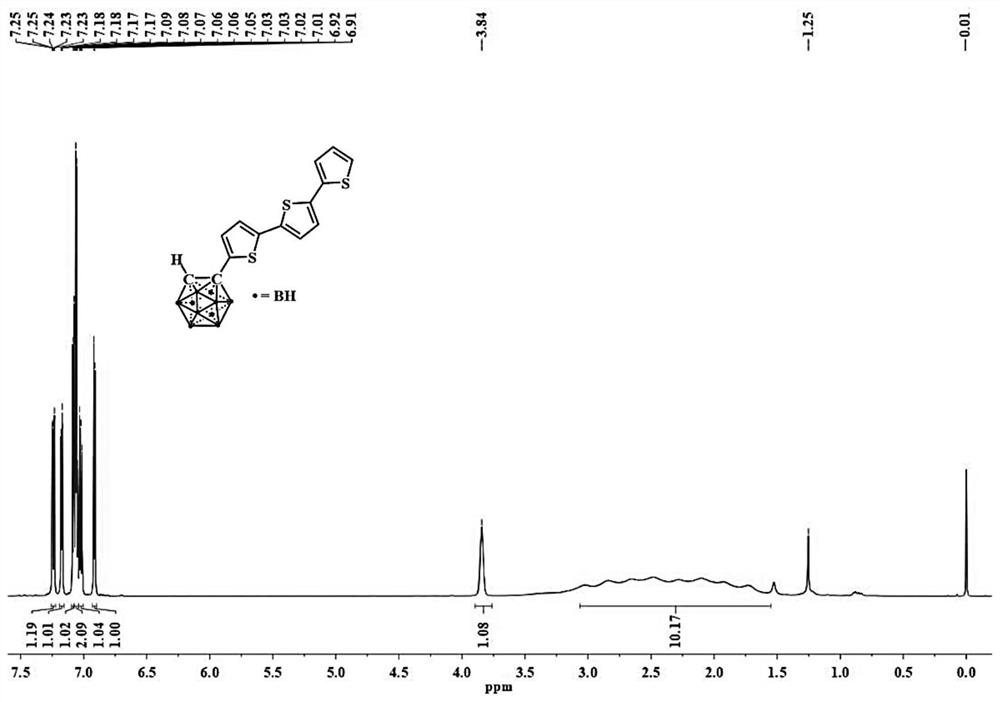
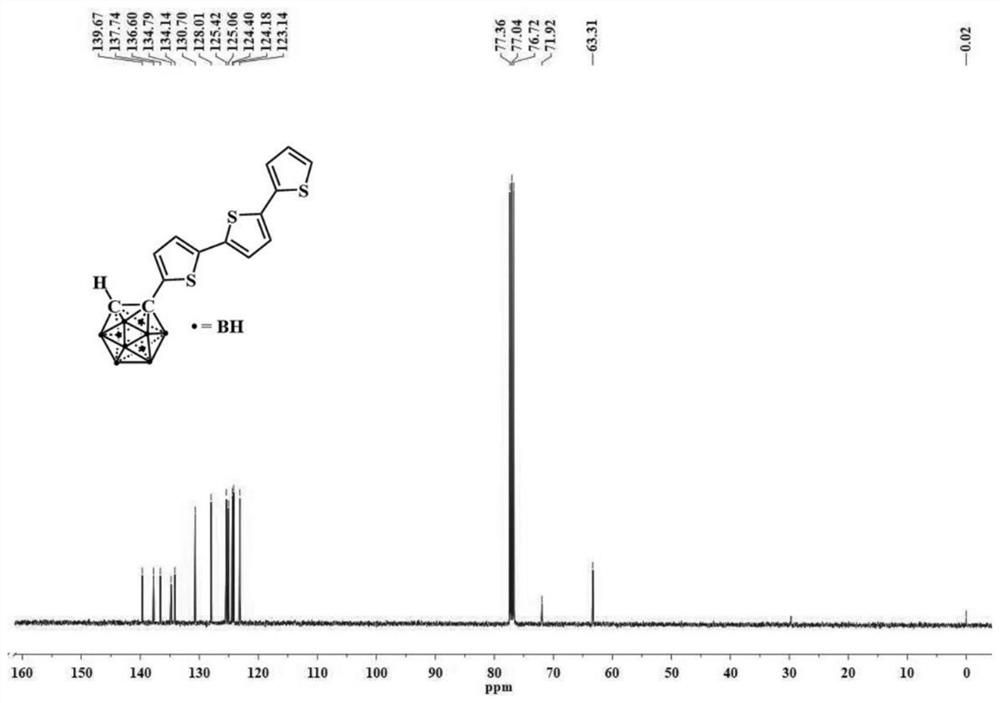
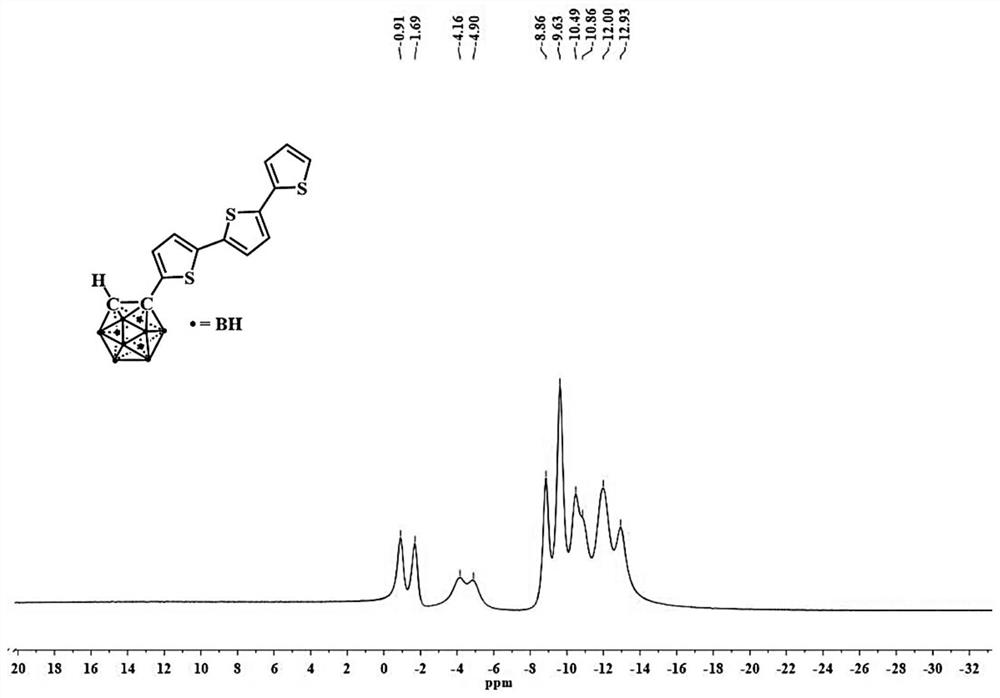
[0058] The structural characterization data of the obtained compound 1 is: 1 H NMR (CDCl 3 ,400MHz)δ:7.23(dd,J=5.1,1.1Hz,1H),7.17(dd,J=3.6,1.1Hz,1H),7.12(d,J=3.8Hz,1H),7.07(s,2H ),7.02(dd,J=5...
Embodiment 2
[0068] 1. Synthesis of compound 3-1
[0069] Under nitrogen protection, weigh 0.27g (1.0mmol) compound 2, 23.1mg (0.02mmol) tetrakis (triphenylphosphine) palladium, 3.8mg (0.02mmol) cuprous iodide and 0.391g (1.2mmol) 5- Bromo-2,2′:5′,2″-tripolythiophene (3T-Br) was placed in a pressure-resistant tube, and 20 mL of triethylamine and 40 mL of tetrahydrofuran were added to the pressure-resistant tube in turn, heated to 90°C, and stirred Reacted for 12 hours, cooled to room temperature, spin-dried, carried out column chromatography separation and drying with dichloromethane and sherwood oil volume ratio 2:1 mixture as eluent, obtained yellow solid compound 3-1.Its reaction equation is as follows:
[0070]
[0071] 2. Synthesis of oligothiophene functionalized ortho carborane derivatives 4-2
[0072] Under nitrogen protection, weigh 0.244g (2.0mmol) decaborane (B 10 h 14 ) into a pressure-resistant tube, add 42 mL of anhydrous toluene and 0.13 mL of N,N-dimethylaniline, stir...
Embodiment 3
[0077] The use of oligothiophene functionalized ortho carborane derivatives obtained in Example 1 and Example 2 as optical limiting materials
[0078] The oligothiophene functionalized ortho-carborane derivatives 4-1 and 4-2 were prepared with THF to a concentration of 1.0×10 - 2 mol / L solution, ultrasonic treatment for 3 to 5 minutes, and the color of the solution is a yellow solution.
[0079] Figure 17 is the two-photon absorption cross-section value of the oligothiophene-functionalized ortho-carborane derivatives 4-1 and 4-2 in the range of 620nm to 850nm. From the figure, we can see that the oligothiophene-functionalized ortho-carborane The maximum two-photon absorption cross section value of alkane derivative 4-1 is 104GM at 650nm, and the nonlinear absorption coefficient can be calculated to be about 2.0×10 -2 cm / GW; the maximum two-photon absorption cross section value of oligothiophene functionalized ortho-carborane derivative 4-2 is 118GM at 650nm, and the nonlin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com