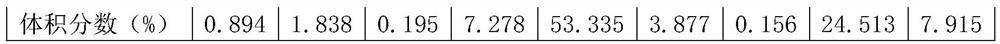Production process and device for preparing high-purity sodium cyanide or potassium cyanide with high yield
A production process, a technology of sodium cyanide, applied in the directions of metal cyanide, simple alkali metal cyanide, etc., can solve the problems of complex operation, large quality gap, low utilization rate of HCN, etc., and achieve the effect of good color and luster
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] In the industrial reaction device, the HCN synthesis gas produced by the Angle method using natural gas, ammonia and air as raw materials, its component content is analyzed in the following table:
[0054] components CH4 NH3 O2 H2 N2 CO CO2 H2O HCN Volume fraction(%) 0.894 1.838 0.195 7.278 53.335 3.877 0.156 24.513 7.915
[0055] The above syngas is absorbed by sulfuric acid in the ammonia removal system to remove ammonia and cooled to 40°C to obtain HCN to remove ammonia. The composition content analysis is as follows:
[0056] components CH4 O2 H2 N2 CO CO2 H2O HCN Volume fraction(%) 1.153 0.251 9.379 68.732 4.187 0.201 5.946 9.342
[0057] The above-mentioned HCN is divided into two parts in addition to ammonia, the first part accounts for 90% of the total volume flow of HCN in addition to ammonia, enters the absorber I, and absorbs continuously at 40 ° C with 32% liquid sodium hydrox...
Embodiment 2
[0066] In the industrial reaction device, the HCN synthesis gas produced by the Angle method using natural gas, ammonia and air as raw materials, its component content is analyzed in the following table:
[0067]
[0068]
[0069] The above syngas is absorbed by sulfuric acid in the ammonia removal system to remove ammonia and cooled to 45°C to obtain HCN ammonia removal gas. The composition content analysis is shown in the following table:
[0070] components CH4 O2 H2 N2 CO CO2 H2O HCN Volume fraction(%) 1.155 0.251 9.397 68.868 4.195 0.201 5.761 9.361
[0071] The above-mentioned HCN is divided into two parts in addition to ammonia, the first part accounts for 75% of the total volume flow of HCN in addition to ammonia, enters the absorber I, and absorbs continuously at 40 ° C with 32% liquid sodium hydroxide, the circulating liquid and the produced The mass ratio of thick sodium cyanide solution is 10:1, and the component content ...
Embodiment 3
[0080] In the industrial reaction device, the HCN synthesis gas produced by the Angle method using natural gas, ammonia and air as raw materials, its component content is analyzed in the following table:
[0081]
[0082] The above syngas is absorbed by sulfuric acid in the ammonia removal system to remove ammonia and cooled to 40°C to obtain HCN to remove ammonia. The composition content analysis is as follows:
[0083] components CH4 O2 H2 N2 CO CO2 H2O HCN Volume fraction(%) 1.153 0.251 9.379 68.732 4.187 0.201 5.946 9.342
[0084] The above-mentioned HCN is divided into two parts in addition to ammonia, the first part accounts for 30% of the total volume flow of HCN in addition to ammonia, enters the absorber I, and absorbs continuously at 40 ° C with 32% liquid sodium hydroxide, the circulating liquid and the produced The mass ratio of thick sodium cyanide solution is 5:1, and the component content analysis of the thick sodium cy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com