Preparation process of pantoprazole sodium for injection
A technology for preparing pantoprazole sodium and its preparation technology, which is applied in the field of preparation technology of pantoprazole sodium for injection, can solve problems such as discoloration and polymerization, inappropriateness, destructive changes in chemical structure, etc., and achieve total impurity and content The effects of small changes, improved stability, and fast reconstitution time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation technology of pantoprazole sodium for injection of the present invention comprises the following steps:
[0045] S100, dosing liquid, comprising the following steps:
[0046] S110. Weighing and mixing: Weigh the prescribed amount of pantoprazole sodium and mannitol, first add 80% of the total amount of dosing, water for injection at 10°C in the liquid mixing tank, and then add the prescribed amount of mannitol and pantoprazole Toprazole sodium, stir to dissolve into a clear solution, then add water for injection to the full amount and mix well;
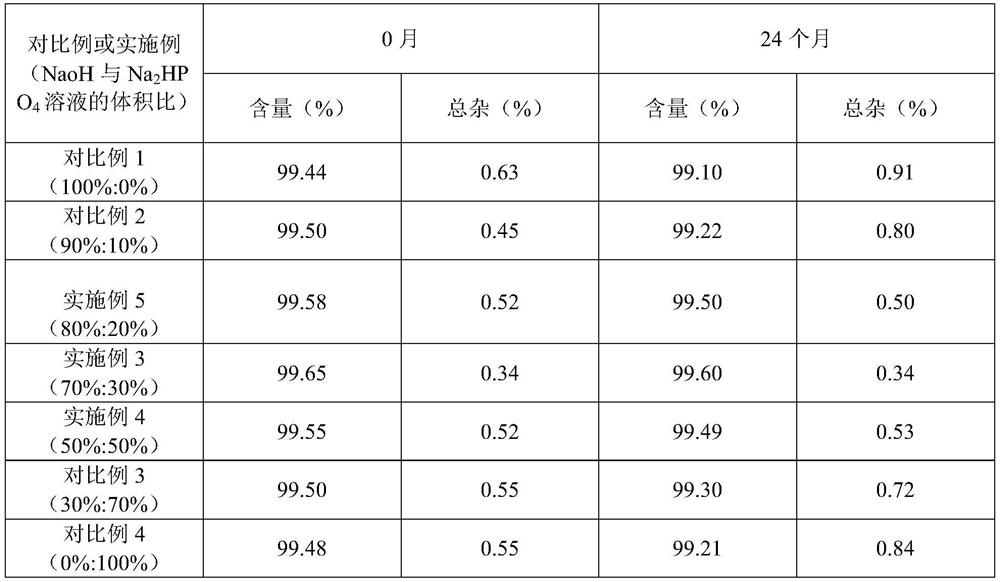
[0047] S120, adjust the pH value: adjust the pH value of the solution to 10.0 with a 5mol / L alkaline reagent; wherein, the alkaline solution is NaoH and Na 2 HPO 4 Mixed solution of NaoH and Na 2 HPO 4 The volume ratio of the solution is 50%:50%;
[0048] S130. Adsorption of impurities: add medicinal activated carbon of 0.10% (g / ml) of the total amount of the solution, stir and adsorb for 30 minutes, then p...
Embodiment 2
[0064] The difference between embodiment 2 and embodiment 1 is:
[0065] (1) The temperature of the water for injection in step S110 is 20°C;
[0066] (2) pH value is adjusted to 12.0 in step S120;
[0067] (3) NaoH and Na in step S120 2 HPO 4 The volume ratio of the solution is 80%: 20%;
[0068] (4) In step S311, the temperature of the shelf is lowered to 5°C.
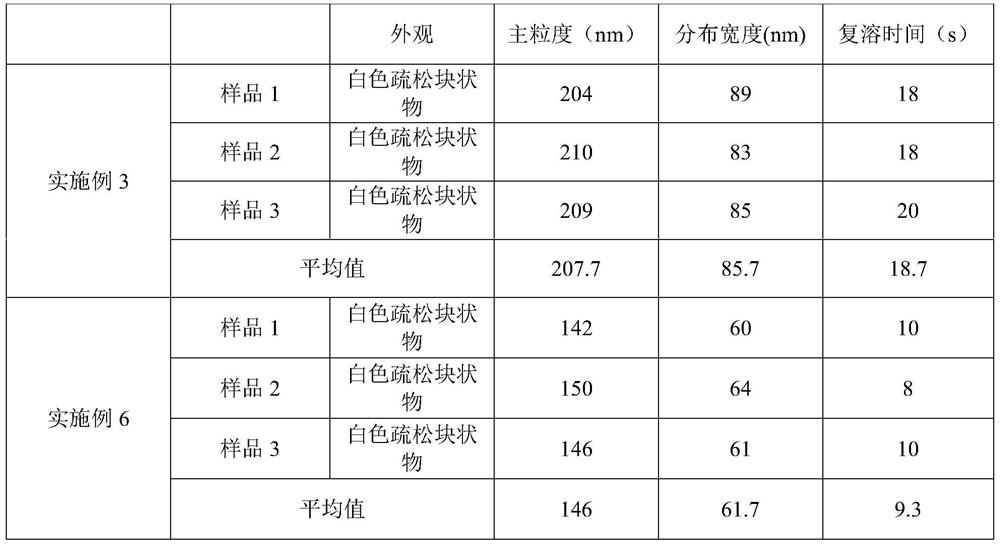
Embodiment 3
[0070] The difference between embodiment 3 and embodiment 1 is:
[0071] (1) The temperature of the water for injection in step S110 is 15°C;
[0072] (2) pH value is adjusted to 11.0 in step S120;
[0073] (3) NaoH and Na in step S120 2 HPO 4 The volume ratio of the solution is 70%: 30%;
[0074] (4) In step S311, the temperature of the shelf is lowered to 10°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com