Polymeric flame retardant as well as preparation method and application thereof
A flame retardant, polymerized technology, used in the field of flame retardants, can solve the problems of environmental and natural environment pollution, flame retardant performance, mechanical properties and heat resistance damage, damage to the mechanical properties of materials, etc., and achieve excellent drip resistance. Falling performance, the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
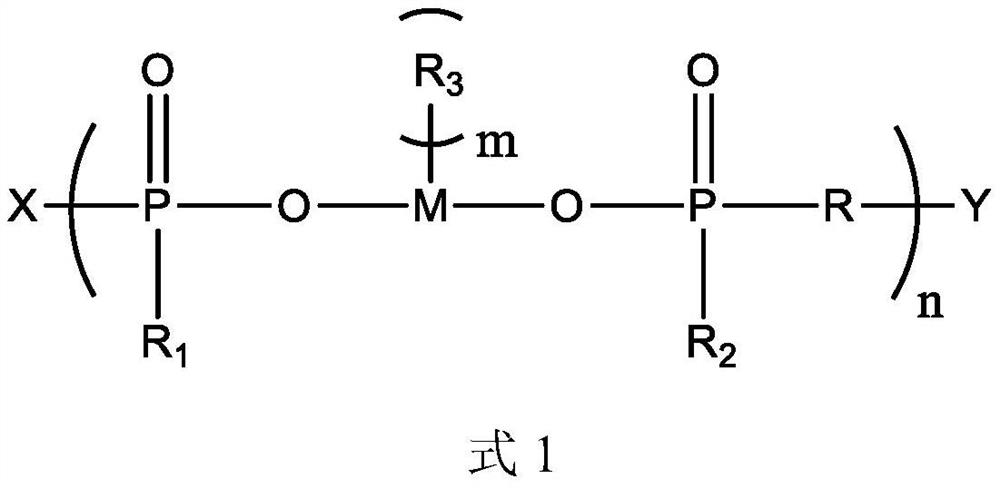
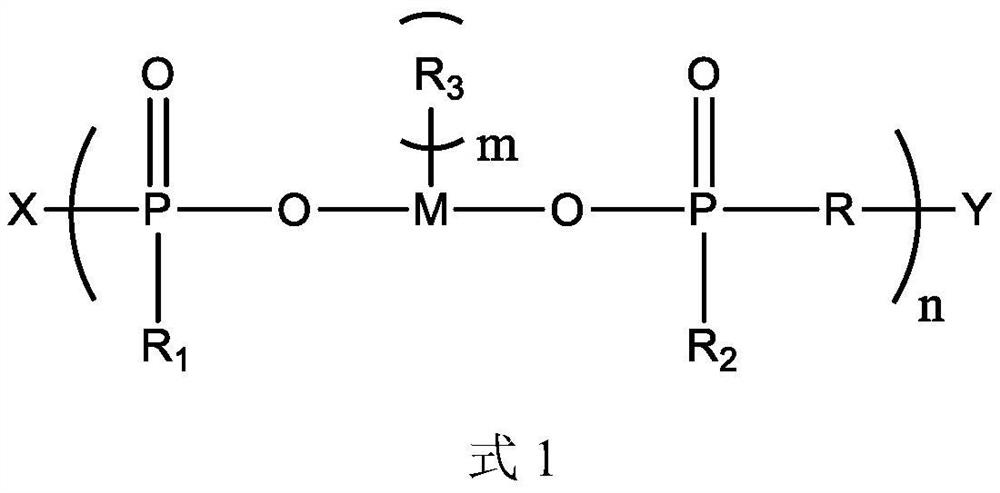
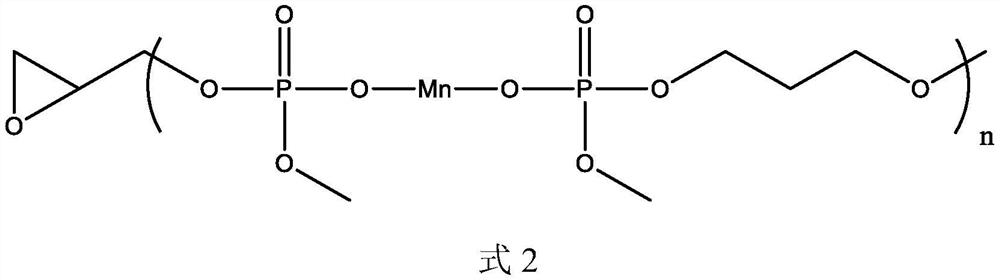
[0056] This embodiment provides a polymeric flame retardant, the structure of which is shown in Formula 2:
[0057]
[0058] The preparation method of the compound shown in formula 2 is: dissolving 1 mol of manganese dihydrogen phosphate in 100 mL of DMF, adding 1 mol of 1,3-propanediol and 0.01 mol of dibutyltin oxide, reacting at 150°C for 3h, 170°C for 3h and React at 190°C for 3 hours. After the solvent is separated by distillation, the product obtained is mixed with 0.95 mol of methanol. After the methanol is completely reacted, 0.1 mol of epichlorohydrin is added. After the reaction, the product is purified to obtain the compound shown in formula 2.
[0059] 1 H NMR (CDCl 3 ,500MHz): δ4.11~4.05(t, 2H, CH 2 ), 3.71~3.63 (s, 6H, CH 3 ), 3.45~3.37(t, 2H, CH 2 ), 1.89~1.82 (m, 2H, CH 2 ).
[0060] No hydroxyl hydrogen peak appears in the result of proton nuclear magnetic resonance spectrum, which proves that the hydroxyl of manganese dihydrogen phosphate has reacted...
Embodiment 2
[0063] This embodiment provides a polymeric flame retardant, the structure of which is shown in formula 3:
[0064]
[0065] The preparation method of the compound shown in formula 3 is: dissolve 1.5mol sodium dihydrogen phosphate and 0.5mol molybdenum trichloride in 100mL NMP, add 0.5mol ethylenediamine and 0.01mol DMAP, react at 160°C for 2.5h, and then react at 175°C After reacting for 2.5 hours and 190° C. for 2.5 hours, the solvent was separated by distillation, and the obtained product was reacted with 2.05 mol of allyl alcohol. After the reaction, the product was purified to obtain the compound shown in formula 3.
[0066] 1 H NMR (CDCl 3 ,500MHz): δ6.02~5.95(m, 2H, HC=CH 2 ), 5.41~5.36(t, 2H, HC=C H 2 ), 5.28~5.21(t, 2H, HC=C H 2 ), 4.59~4.52 (d, 4H, CH 2 ), 2.83~2.75 (m, 4H, CH 2 ), 2.30~2.22 (t, 2H, NH).
[0067] No hydroxyl hydrogen peaks appeared in the results of the proton nuclear magnetic resonance spectrum, which proved that the hydroxyl groups of t...
Embodiment 3
[0069] This embodiment provides a polymeric flame retardant, the structure of which is shown in Formula 4:
[0070]
[0071] The preparation method of the compound shown in Formula 4 is: dissolve 1 mol of zinc dihydrogen phosphate in 100 mL of DMSO, add 1 mol of 1,4-cyclohexanediamine and 0.01 mol of DMAP, react at 190°C for 3h, 210°C for 3h and 230°C After reacting at ℃ for 3 hours, the solvent was separated by distillation, and the obtained product was reacted with 3 mol of epichlorohydrin. After the reaction, it was acidified, washed with water until neutral, and the product was purified to obtain the compound shown in formula 4.
[0072] 1 H NMR (CDCl 3 ,500MHz): δ4.13~4.06(t, 4H, CH 2 ), 3.72~3.65(t, 2H, OH), 3.53~3.46(m, 4H, CH 2 ), 2.58~2.52(m, 2H, CH), 2.30~2.22(d, 2H, NH), 2.01~1.95(m, 4H, CH 2 ), 1.75~1.69 (m, 2H, CH 2 ), 1.62~1.55 (m, 2H, CH 2 ), 1.51~1.44 (m, 2H, CH 2 ), 1.36~1.30 (m, 2H, CH 2 ).
[0073] The results of the hydrogen nuclear magnetic res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com