Method for separating and determining 3-amino-4-imino rifamycin S and related impurities
A technology for rifamycin and related impurities, which is applied in the field of analytical chemistry, can solve the problem that the determination method of 3-amino-4-imino-rifamycin has not been queried, and achieves an effective method, simple operation and short analysis time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
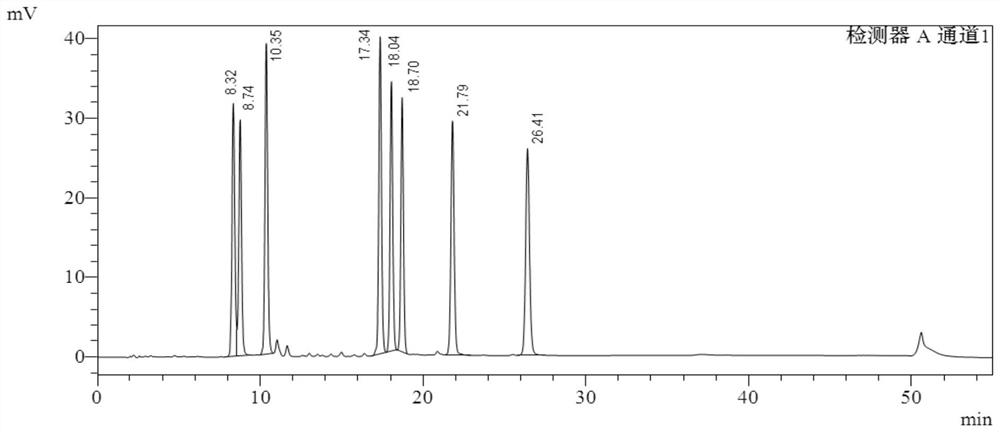
Image
Examples
Embodiment 1
[0074] Supplies and methods
[0075] 3-amino-4-iminorifamycin S standard, 3-aminorifamycin S, and rifamycin S standard were all obtained from commercial channels. The sources of 3-amino-4-iminorifamycin S for testing and 3-bromorifamycin S, impurity A, impurity B, impurity C, and impurity D are from Chongqing Hua Bangshengkai Pharmaceutical Co., Ltd.
[0076] The test instruments used are Shimadzu high performance liquid chromatography (UV detector)
[0077] The chromatographic column is ZORBAX Eclipse XDB-C18, 4.6×250mm, 5-Micron; the column temperature is 30°C; the injection volume is 15μl; the flow rate is 1.0ml / min; the sample concentration is 0.4mg / ml.
[0078] Mobile phase A: the volume ratio of acetonitrile and methanol is 900:100;
[0079] Mobile phase B: a mixed solution of ammonium dihydrogen phosphate buffer and acetonitrile, the volume ratio is 800:200, the concentration of ammonium dihydrogen phosphate solution is 0.1mol / L, and the pH value is 6-8.5.
[0080] ...
Embodiment 2
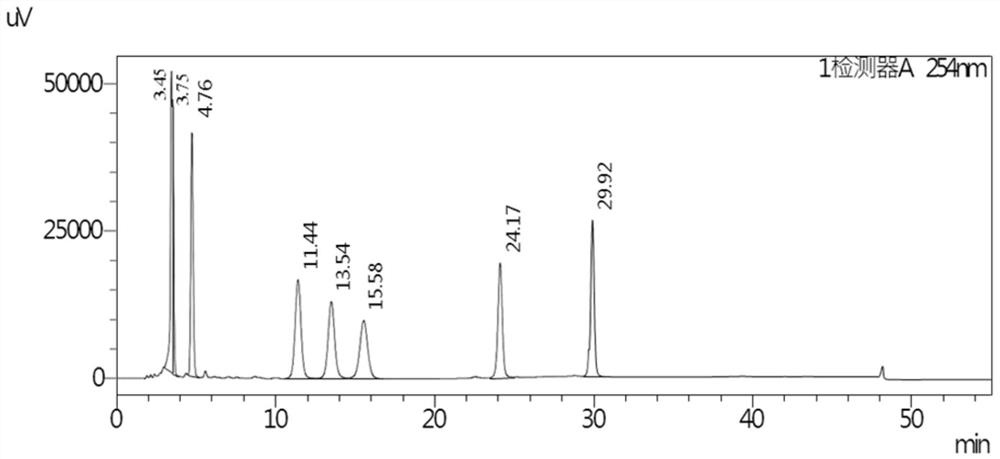
[0092] The buffer salt used is sodium dihydrogen phosphate aqueous solution, pH is 6.0, gradient elution, the results are shown in the appendix figure 1 .
Embodiment 3
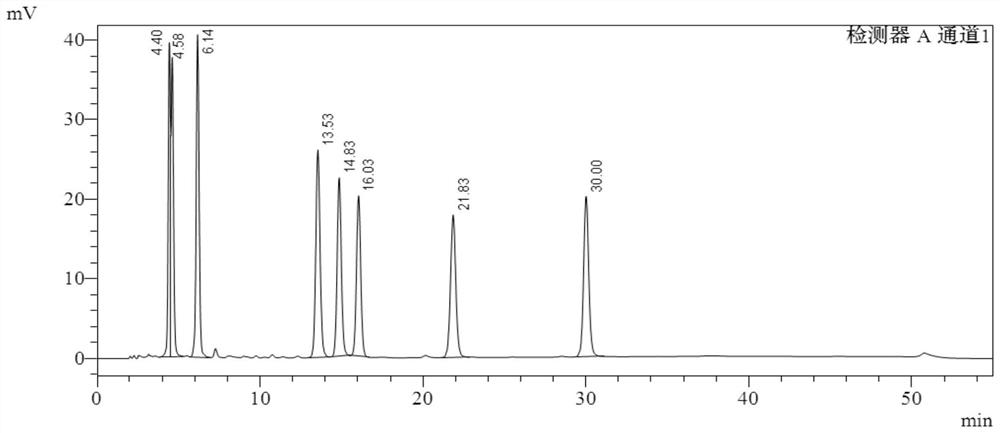
[0094] The buffer salt used is potassium dihydrogen phosphate aqueous solution, the pH is 6.0, and the results of gradient elution are shown in the appendix figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com