Leonurine hydrochloride tablet and preparation method thereof
A technology of leonurine hydrochloride and tablets, which can be used in pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc. It can solve problems such as unacceptable and large dosage for patients, and achieve stable quality, avoid residues, and cheap raw materials Easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
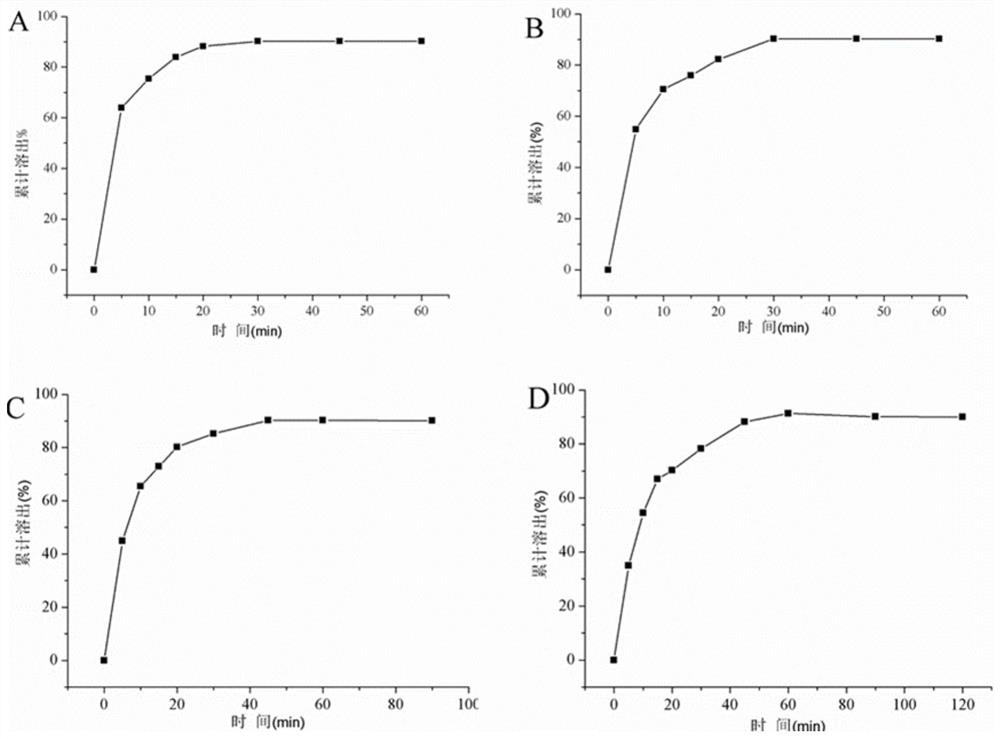
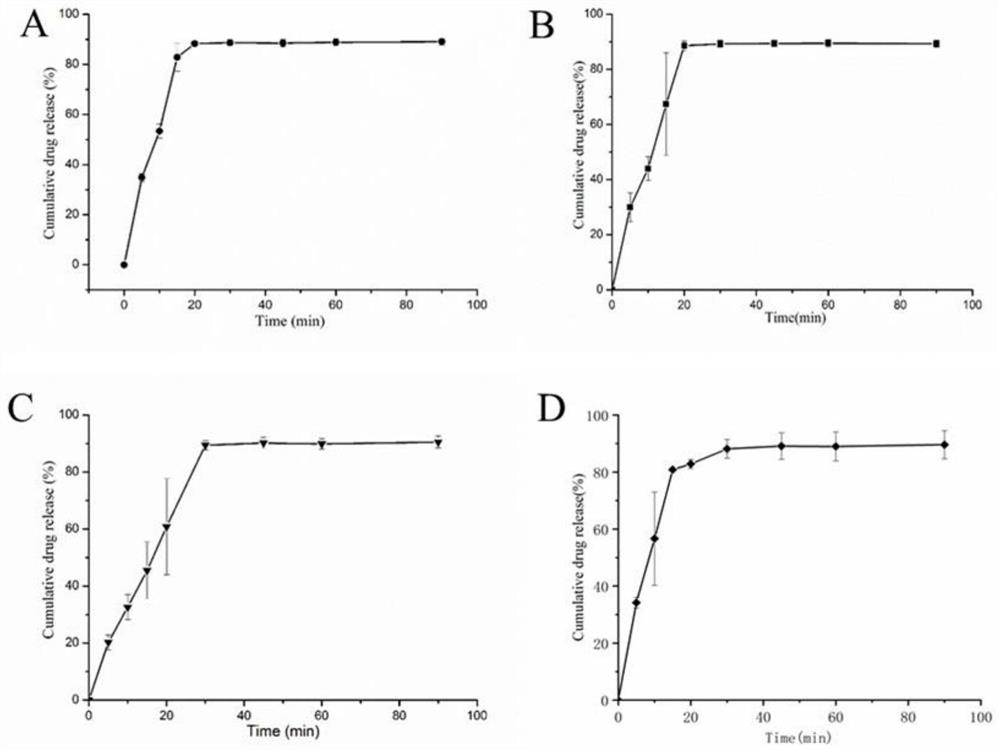
[0051]Considering the large proportion of auxiliary materials, starch, sucrose, microcrystalline cellulose, etc. are now used as fillers. The proportion of other excipients remains unchanged, of which the main drug accounts for 5%, the disintegrant accounts for 5%, and the lubricant accounts for 0.5%. The fluidity of the granules, compression formability, hygroscopicity, economic cost, tablet appearance, disintegration time, etc. were used as the investigation indicators to compare the differences among the formulations.
[0052] Prescription 1: The filling agent is 8950mg starch, 500mg leonurine hydrochloride, 500mg crospovidone, and 50mg magnesium stearate.
[0053] Prescription 2: The filler is 8950 mg of sucrose, 500 mg of motherurine hydrochloride, 500 mg of crospovidone, and 50 mg of magnesium stearate.
[0054] Prescription 3: The filler is 8950 mg of microcrystalline cellulose, 500 mg of motherurine hydrochloride, 500 mg of crospovidone, and 50 mg of magnesium stearat...
Embodiment 2
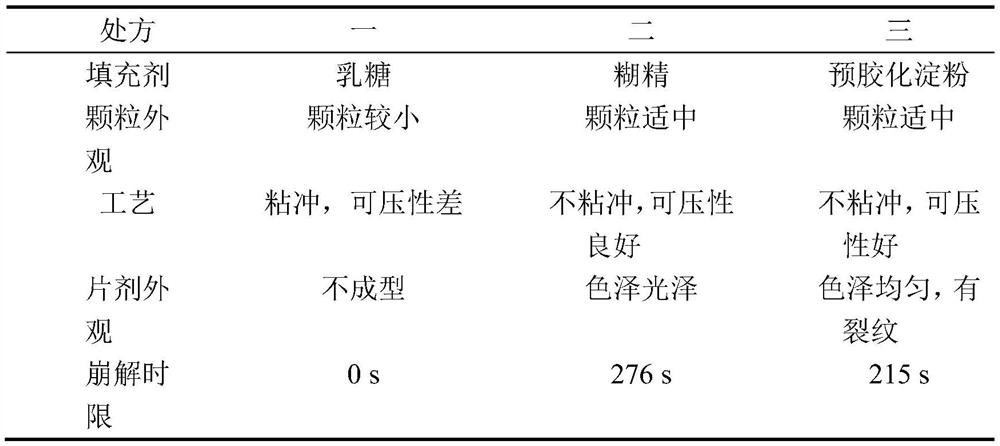
[0057] Considering the large proportion of excipients, lactose, dextrin, pregelatinized starch, etc. are now used as fillers. The proportion of other excipients remains unchanged, of which the main drug accounts for 5%, the disintegrant accounts for 5%, and the lubricant accounts for 0.5%. Taking whether the granules are easy to form, whether they are sticky and punchable during tablet compression, compressibility, tablet appearance, and disintegration time limit, etc., are used as inspection indicators to compare the differences in various formulations and determine the appropriate type of filler.
[0058] Prescription 1: The filler is lactose 8950mg, leonurine hydrochloride 500mg, crospovidone 500mg, magnesium stearate 50mg;
[0059] Prescription 2: The filler is dextrin 8950mg, motherurine hydrochloride 500mg, crospovidone 500mg, magnesium stearate 50mg;
[0060] Prescription 3: The filler is 8950 mg of pregelatinized starch, 500 mg of motherurine hydrochloride, 500 mg of ...
Embodiment 3
[0063] In order to investigate the effects of different types of disintegrants on the preparation process and disintegration time of prescription tablets, dry starch and low-substituted hydroxypropyl cellulose were used as disintegrants. In combination with the filler screened in Example 2, the content of other adjuvants remains unchanged. The economic cost, whether the granules are easy to form, whether they are sticky and punchable during tablet compression, compressibility, tablet appearance, and disintegration time limit are used as the inspection indicators. Compare the differences in each formulation to determine the appropriate type of disintegrant.
[0064] Prescription 1: Leonurine hydrochloride 500mg, disintegrating agent is dry starch 500mg, magnesium stearate 50mg.
[0065] Prescription 2: The disintegrating agent is 500 mg of low-substituted hydroxypropyl cellulose, 500 mg of motherwortine hydrochloride, and 50 mg of magnesium stearate.
[0066] The above two pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com