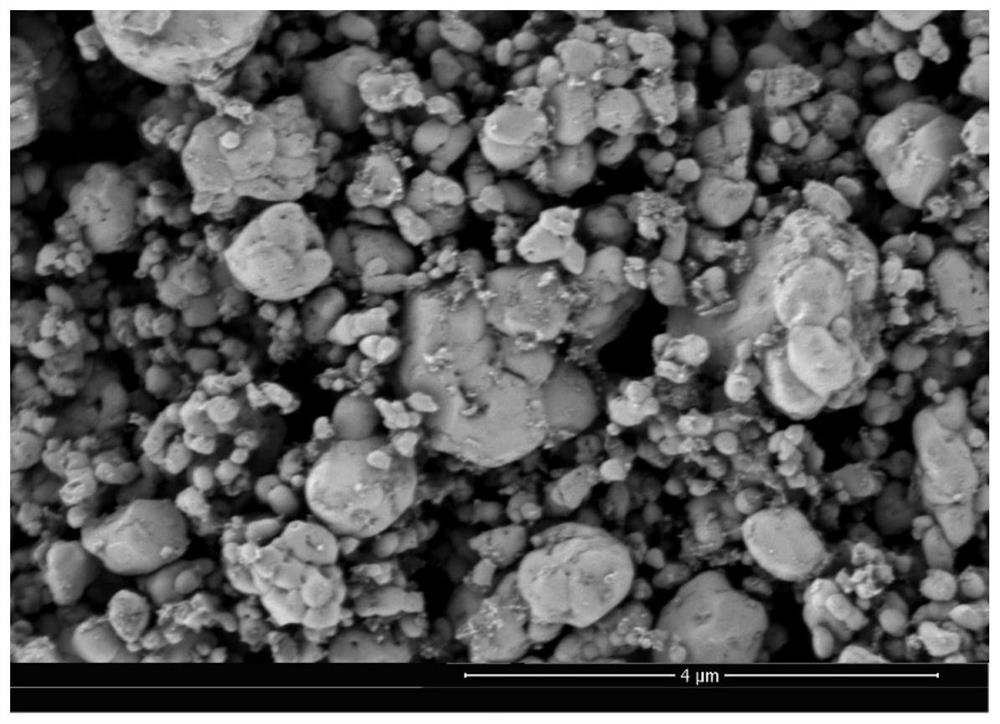
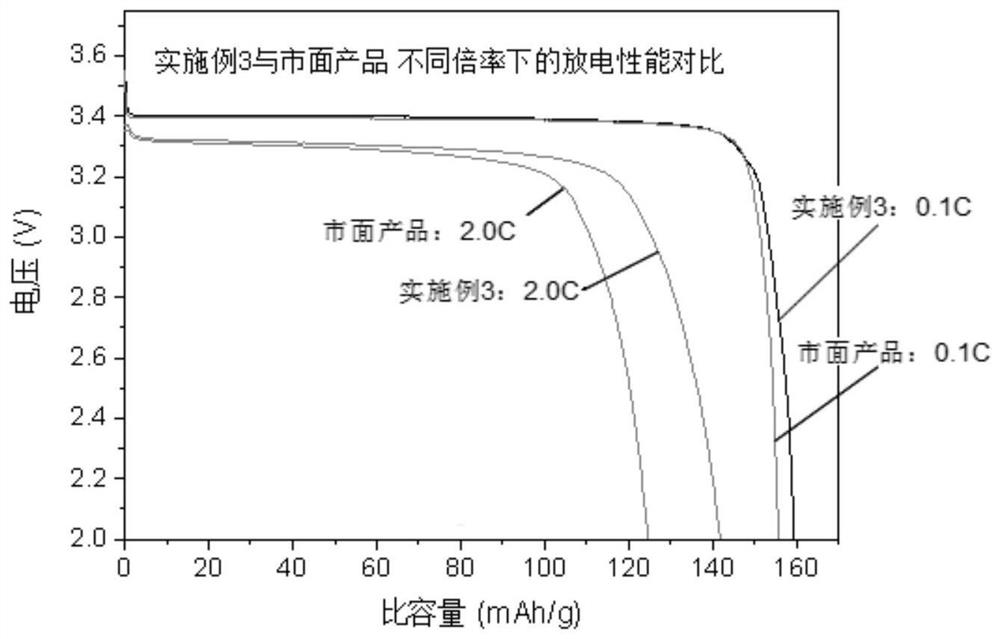
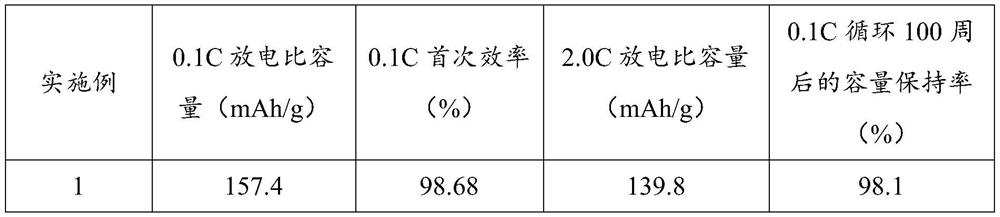
Preparation method and application of lithium iron phosphate positive electrode material
A positive electrode material, lithium iron phosphate technology, applied in the field of preparation of lithium iron phosphate positive electrode material, can solve the problems of poor rate performance, poor performance, and many control points, to achieve performance optimization and improvement, easy to operate the preparation process, and the preparation process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A preparation method of lithium iron phosphate cathode material, comprising the following steps:
[0034] (1) Ferric oxalate, ammonium dihydrogen phosphate, and lithium hydroxide are 1:1.03:1.07 according to the molar ratio of Fe:P:Li, weigh the weight, then add 4.5% glucose and 2.36% polyacrylic acid as carbon source, the additive is 0.08% titanium oxide and 0.10% barium nitrate are mixed, and the particle size (D50) of the mixed material obtained after mixing and refining by a high-efficiency mixer is 35 μm;
[0035] (2) Heat the mixed material at a rate of 3°C / min to 650°C in a pure nitrogen atmosphere for sintering and heat preservation for 5 hours, and perform airflow crushing after discharging, and the crushing particle size (D50) is 1.2 μm;
[0036] (3) The pulverized material is subjected to secondary sintering, and the temperature is raised to 720°C at a rate of 5°C / min for sintering and heat preservation for 8 hours. During this period, 140g / kg of ethanol is i...
Embodiment 2
[0038] A preparation method of lithium iron phosphate cathode material, comprising the following steps:
[0039] (1) Ferric phosphate, lithium carbonate, and ammonium monohydrogen phosphate are 1:1.04:1.09 according to the molar ratio of Fe:P:Li, weigh the weight, then add 6.5% sucrose and 4.6% polyvinyl alcohol as the carbon source, and the additive is 0.24% ammonium metavanadate and 0.22% chromium nitrate nonahydrate are mixed, and the particle size (D50) of the obtained mixed material after mixing and refining by a high-efficiency mixer is 23 μm;
[0040] (2) The mixed material was heated up to 690°C at a rate of 4°C / min in a pure nitrogen atmosphere for sintering and heat preservation for 6 hours. After discharging, it was jet-milled, and the crushed particle size (D50) was 1.4 μm;
[0041] (3) The pulverized material is subjected to secondary sintering, and the temperature is raised to 740°C at a rate of 6.5°C / min for sintering and heat preservation for 8 hours. During th...
Embodiment 3
[0043] A preparation method of lithium iron phosphate cathode material, comprising the following steps:
[0044] (1) Iron phosphate and lithium hydroxide are weighed according to Fe:P:Li molar ratio of 1:1.06:1.10, then add 5.5% sucrose, 3.5% polyethylene glycol, 1.2% oxalic acid as carbon source, additive 0.13% titanium oxide and 0.16% magnesium oxide are mixed, and the particle size (D50) of the obtained mixed material after mixing and refining by a high-efficiency mixer is 55 μm;
[0045](2) Raise the temperature of the mixed material to 670°C at a rate of 5°C / min in a pure nitrogen atmosphere for sintering and heat preservation for 8 hours, and perform airflow crushing after discharging, and the crushing particle size (D50) is 0.8 μm;
[0046] (3) The pulverized material is subjected to secondary sintering, and the temperature is raised to 745°C at a rate of 8°C / min for sintering and heat preservation for 6 hours. During this period, 125g / kg of methanol is introduced, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com