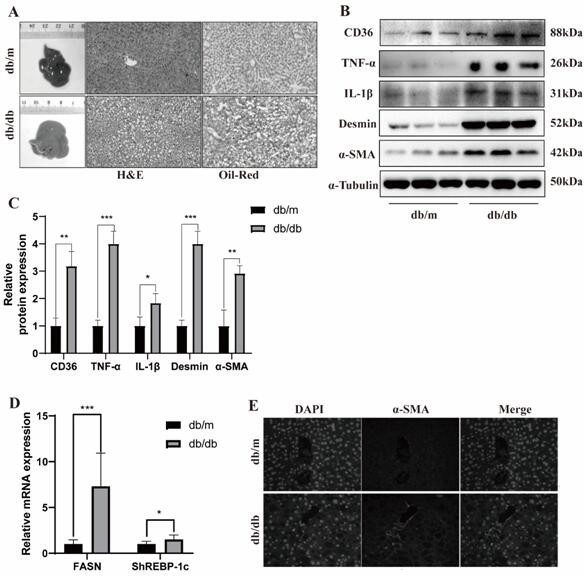
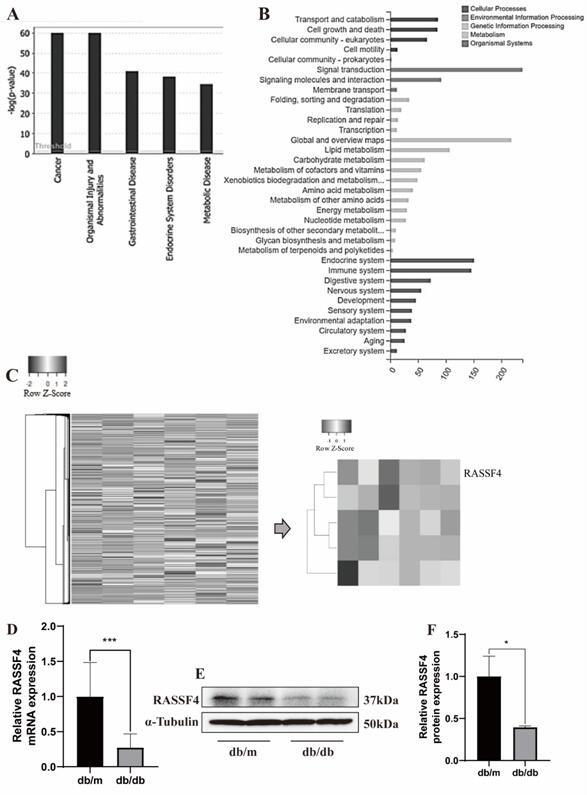
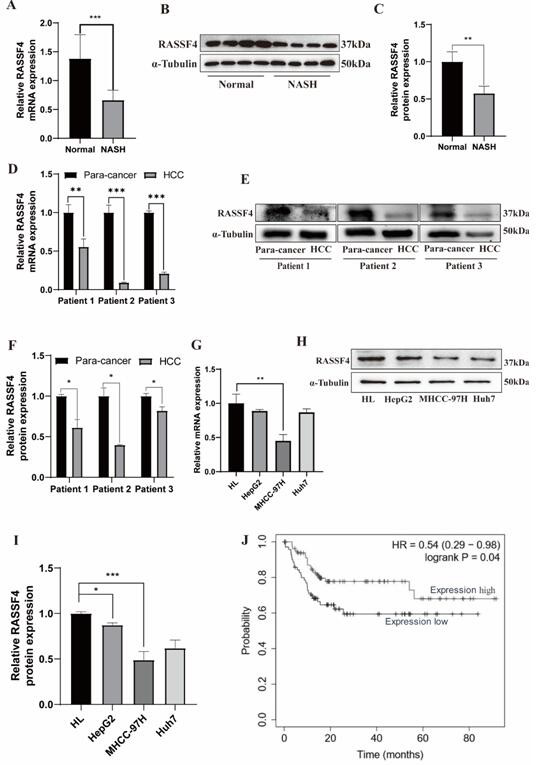
Rassf4 as a target and application in the treatment of diabetes mellitus with non-alcoholic fatty liver disease and liver cancer
A fatty liver disease, non-alcoholic technology, applied in the field of bioengineering of drug preparation, can solve the problems of difficult treatment, unsatisfactory effect, targeted intervention, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] RASSF4 overexpression vector plasmid construction
[0065] 1.1 PCR amplification of RASSF4
[0066] (1) Prepare the following system in a PCR tube:
[0067]
[0068] (2) After the above system is configured, the centrifuge is centrifuged, and PCR equipment is used for amplification; the reaction conditions are as follows:
[0069] Pre-denaturation at 95°C for 5 minutes, denaturation at 95°C for 10 seconds, annealing at 62°C for 30 seconds, extension at 72°C for 2 minutes, 40 cycles;
[0070] (3) Add the sample to 6×DNA loading, and add it to the prepared 1% agarose gel, DNA electrophoresis instrument 150V, about 15 minutes, take the gel after electrophoresis to the dark room, and irradiate it under ultraviolet light Next, try to cut off the gel containing only the target band and put it into a clean 1.5ml EP tube.
[0071] 1.2 Cut rubber recycling
[0072] (1) Weigh the weight of the cut gel and add 300ul BM Buffer to 100mg gel;
[0073] (2) Heat the gel with Bu...
Embodiment 2
[0107] With RASSF4 as the active ingredient, adding pharmaceutically acceptable auxiliary materials, it can be prepared into liquid injections of various specifications according to conventional methods. There are various administration routes of RASSF4, such as injection administration, intracavitary administration and so on.
[0108] (1) Preparation of injections:
[0109] RASSF4 200 mg, mannitol 700 mg, PEG3000 10 mg, distilled water 100 ml, make the pH value 7.0-7.5, filter the filtrate to a concentration of 3 mg / ml, pack in 2 ml per ampoule, and freeze-dry to obtain the injection.
[0110] (2) Preparation of tablets:
[0111] RASSF4 10 mg, microcrystalline cellulose 35 mg, starch 45 mg, polyvinylpyrrolidone 4 mg, carboxymethyl starch sodium salt 4.5 mg, magnesium stearate 0.5 mg, talc 1 mg; RASSF4 active ingredient, starch and fiber sieve and mix thoroughly, mix the polyvinylpyrrolidone solution with the above powder, sieve to obtain wet granules and dry at 50°C, pre-si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com