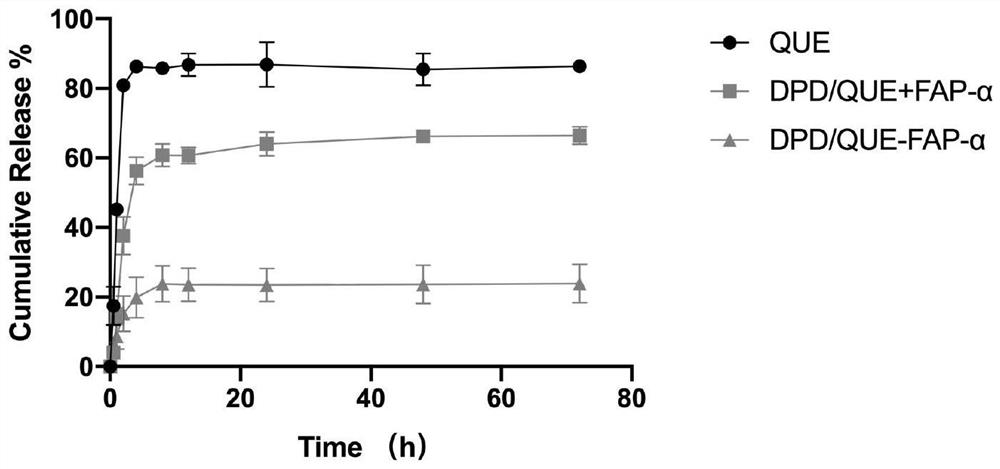
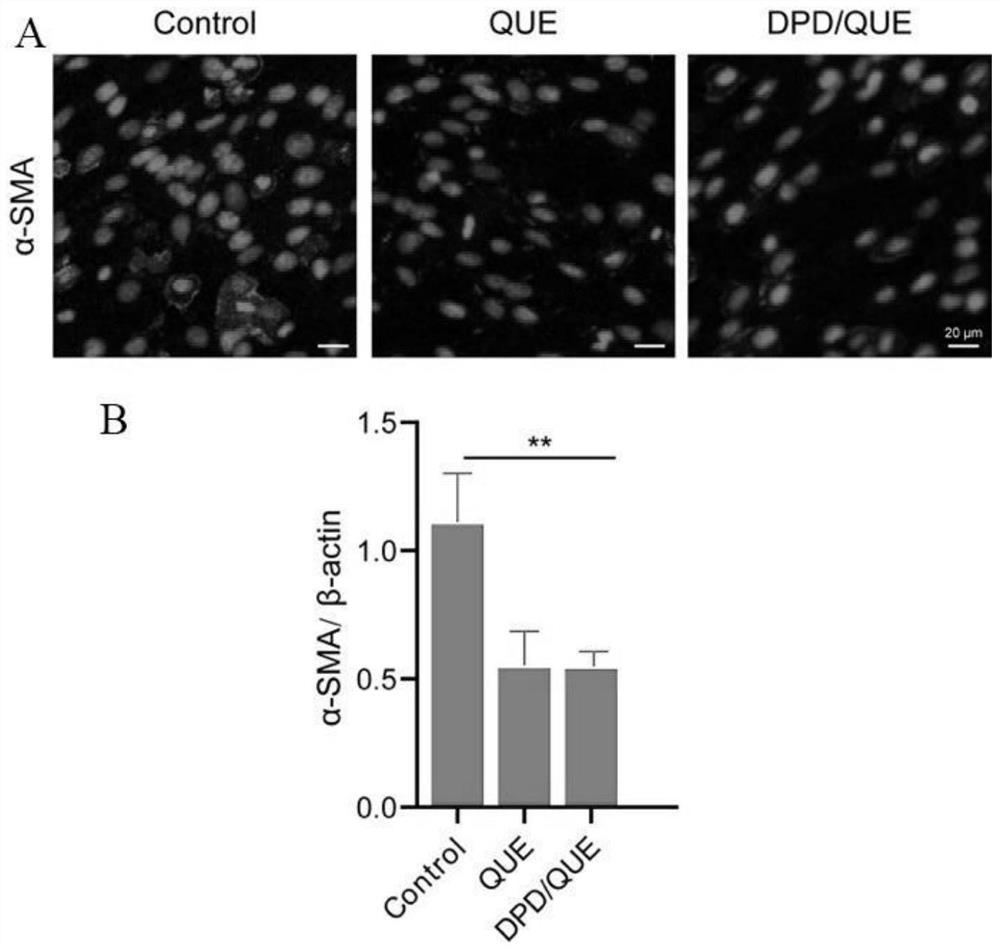
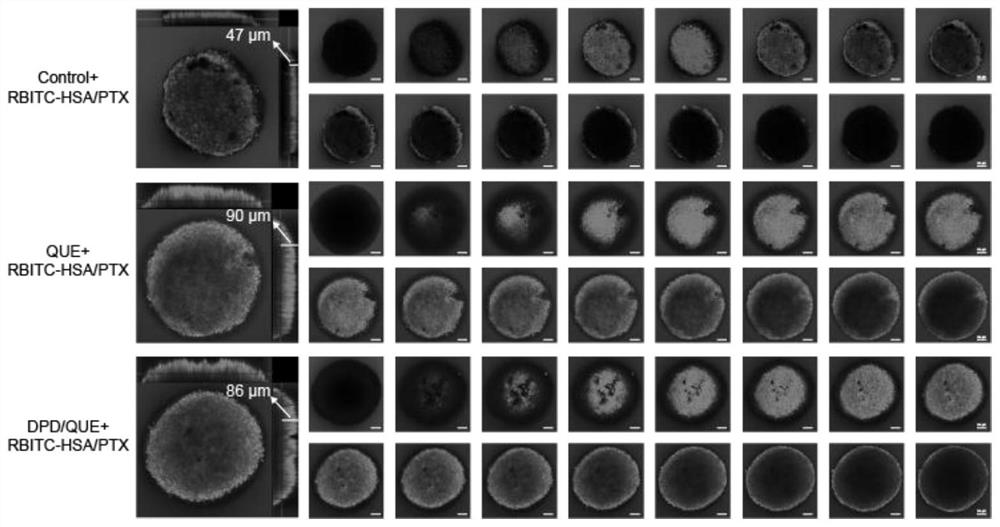
Preparation and application of amphiphilic glucan derivative carrier targeting tumor-associated fibroblasts and its pharmaceutical composition
A technology related to fibroblasts and tumors, which is applied in the field of preparation and application of amphiphilic dextran derivative carriers and their pharmaceutical compositions, and can solve problems such as lack of responsiveness, slow drug release speed, and hindrance to drug efficacy , to achieve the effects of improving solubility, promoting penetration, good biocompatibility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Carboxylated dextran-FAPα-responsive peptide-octadecylamine (Dex-peptide-C 18 ) is prepared as follows:
[0045] (1) Dissolve 2000 mg of dextran in dimethyl sulfoxide to obtain a dextran solution; add 380 mg of glutaric anhydride and 10 mg of DMAP into the dextran solution, and react at room temperature for 24 hours. The reaction solution was added dropwise to excess ice ethanol to form a precipitate. The collected precipitate was washed three times with ice ethanol, redissolved in water, dialyzed (MWCO=7000) for 72 hours, and freeze-dried to obtain the carboxylated dextran intermediate (Dex- COOH);
[0046] (2) Take 132 mg of Boc-Ser-Gly-Pro, a peptide linker protected by Boc, and dissolve it in 10 mL of DMF; add 108 mg of octadecylamine, 55 mg of HOBT and 77 mg of EDC successively, react for 24 hours, add an appropriate amount of water to mix with the reaction solution, and extract the product with ethyl acetate , washed three times with dilute hydrochloric acid, so...
Embodiment 2
[0049] The preparation method of carboxylated dextran-FAPα response peptide-deoxycholic acid (Dex-peptide-DOCA) is as follows:
[0050] (1) Dissolve 2000 mg of dextran in dimethyl sulfoxide to obtain a dextran solution; add 334 mg of succinic anhydride and 10 mg of DMAP into the dextran solution, and react at room temperature for 24 hours. The reaction solution was added dropwise to excess ice ethanol to form a precipitate. The collected precipitate was washed three times with ice ethanol, redissolved in water, dialyzed (MWCO=7000) for 72 hours, and freeze-dried to obtain the carboxylated dextran intermediate (Dex- COOH);
[0051] (2) Dissolve 1000 mg of deoxycholic acid (DOCA) in 20 mL of tetrahydrofuran, add 381 mg of NHS and 628 mg of DCC respectively, react for 12 hours, remove the white precipitate by suction filtration, add excess cyclohexane to produce a precipitate, and collect the white precipitate by suction filtration to obtain deoxygenation Cholic acid-activated e...
Embodiment 3
[0055] Carboxylated dextran-FAPα-responsive peptide-laurylamine (Dex-peptide-C 12 ) is prepared as follows:
[0056] (1) Dissolve 2000 mg of dextran in dimethyl sulfoxide to obtain a dextran solution; add 427 mg of adipic anhydride and 10 mg of DMAP into the dextran solution, and react at room temperature for 24 hours. The reaction solution was added dropwise to excess ice ethanol to form a precipitate. The collected precipitate was washed three times with ice ethanol, redissolved in water, dialyzed (MWCO=7000) for 72 hours, and freeze-dried to obtain the carboxylated dextran intermediate (Dex- COOH);
[0057] (2) Take 167 mg of Boc-Ile-Gly-Pro-Ala, a peptide linker protected by Boc, and dissolve it in 10 mL of DMF; add 74 mg of laurylamine, 55 mg of HOBT and 77 mg of EDC successively, react for 24 hours, add an appropriate amount of water to mix with the reaction solution, and extract with ethyl acetate The product was washed three times with dilute hydrochloric acid, sodiu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com