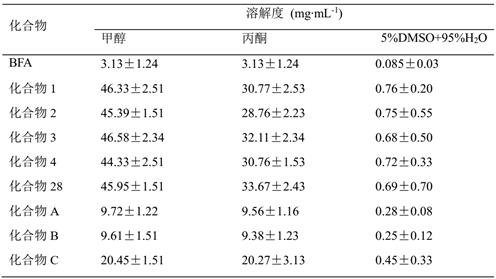
Application of brefeldin A ester derivative in antitumor drug
A technology of feldspar and ester compounds, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
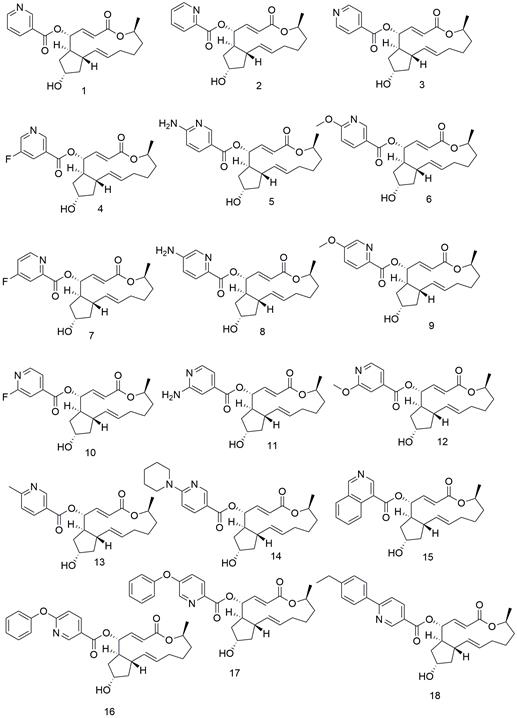
[0160] Example 1: 4-O-Nicotinyl BFA (Compound 1)
[0161]
[0162] Under the protection of nitrogen, BFA (40mg, 1eq), DMAP (1eq) and EDC·HCl (4eq) were dissolved in 8ml of anhydrous DCM, stirred at 40°C for 10 min, then added the DCM solution of niacin (2eq) to continue the reaction After 2 hours, after the reaction was detected by TLC, water was added for extraction, the organic phase was concentrated under reduced pressure, and the crude extract was separated and purified to obtain a white solid with a yield of 40%. 1 H NMR (400 MHz, Chloroform-d) δ= 9.23 (dd, J=2.2, 0.9, 1H), 8.78 (dd, J=4.9, 1.7, 1H), 8.30 (dt, J=8.0, 2.0,1H) , 7.40 (ddd, J=7.9, 4.9, 0.9, 1H), 7.32 (dd, J=15.7, 3.4, 1H), 5.79 – 5.66(m, 2H), 5.51 (ddd, J=10.5, 3.4, 1.9, 1H), 5.33 (dd, J=15.2, 9.5, 1H), 4.83(dqd, J=10.9, 6.2, 1.8, 1H), 4.32 (tt, J=5.3, 2.5, 1H), 2.48 (qd, J= 9.2, 6.7,1H), 2.35 (tt, J=10.5, 8.2, 1H), 2.23 (ddd, J=14.1, 9.3, 5.1, 1H), 2.12 (d, J=3.1, 1H), 2.05 – 1.94 ( m, 2H), 1.90 – 1.7...
Embodiment 2
[0163] Example 2: 4-O-(2-pyridinecarboxylic acid) acyl BFA (Compound 2)
[0164]
[0165] The preparation method of Reference Example 1 obtained a white solid with a yield of 45%. 1 H NMR (400 MHz, Chloroform-d)δ = 8.82 – 8.74 (m, 1H), 8.17 – 8.08 (d, J=7.8, 1H), 7.90 – 7.80 (td, J=7.8,1.7, 1H), 7.53 – 7.45 (dd, J=7.7, 4.8, 1H), 7.39 – 7.28 (dd, J=15.7, 3.3,1H), 5.86 – 5.77 (dt, J=15.7, 1.7, 1H), 5.77 – 5.67 (ddd, J=14.9, 10.1, 4.5,1H), 5.64 – 5.55 (ddd, J=10.3, 3.4, 1.7, 1H), 5.38 – 5.26 (dd, J=15.2, 9.2,1H), 4.88 – 4.77 (m, 1H ), 4.37 – 4.27 (p, J=4.6, 1H), 2.55 – 2.35 (ddd, J=31.1, 13.8, 8.4, 2H), 2.28 – 2.17 (ddd, J=14.1, 9.0, 5.2, 1H), 2.07 – 1.99(m, 2H), 1.89 – 1.82 (dd, J=11.4, 4.5, 2H), 1.77 – 1.66 (dt, J=13.3, 8.4,2H), 1.59 – 1.47 (m, 2H), 1.25 – 1.21 (d, J=6.3, 3H), 1.00 – 0.87 (ddt, J=17.8, 10.2, 4.6, 1H). 13 C NMR (100 MHz, Chloroform-d) δ 165.7, 164.0, 150.2, 147.5, 146.9, 137.2, 136.5, 130.8, 127.3, 125.4, 118.6, 77.9, 72.4, 72.0, 41, 4.25, 3.9 , 26.7, 20...
Embodiment 3
[0166] Example 3: 4-O-(4-pyridinecarboxylic acid) acyl BFA (Compound 3)
[0167]
[0168] The preparation method of Reference Example 1 obtained a white solid with a yield of 47%. 1 H NMR (400 MHz, Chloroform-d)δ = 8.86 – 8.69 (d, J=5.0, 2H), 7.93 – 7.82 (m, 2H), 7.36 – 7.28 (dd, J=15.7,3.4, 1H), 5.80 – 5.66 (m, 2H), 5.57 – 5.48 (ddd, J=10.5, 3.4, 1.8, 1H), 5.40– 5.28 (dd, J=15.2, 9.5, 1H), 4.90 – 4.78 (dqd, J=12.5, ( ddd, J=14.3, 9.4, 5.2, 1H), 2.07 – 1.94 (m, 2H), 1.93 –1.80 (m, 3H), 1.79 – 1.61 (dddd, J=28.3, 14.0, 9.2, 3.5, 2H), 1.59 – 1.46 (m,2H), 1.29 – 1.20 (d, J=6.3, 3H), 1.00 – 0.87 (m, 1H). 13 C NMR (100 MHz, Chloroform-d) δ 165.6, 164.1, 150.8, 146.5, 136.9, 136.4, 131.0, 123.0, 118.7, 78.0, 72.4, 72.2, 49.8, 44.5, 43.3, 41.2, 20.2, 34. .HRESIMS m / z 386.1957 [M+H] + (calcd. for [C 22 h 28 o 5 N] + , 386.1962).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com