Sodium bromide application experiment method
An experimental method and sodium bromide technology are applied in the field of valsartan intermediate synthesis product processing, can solve problems such as environmental pollution, waste of bromine elements, sodium bromide treatment, etc., and achieve the effects of reducing costs and reducing environmental protection emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
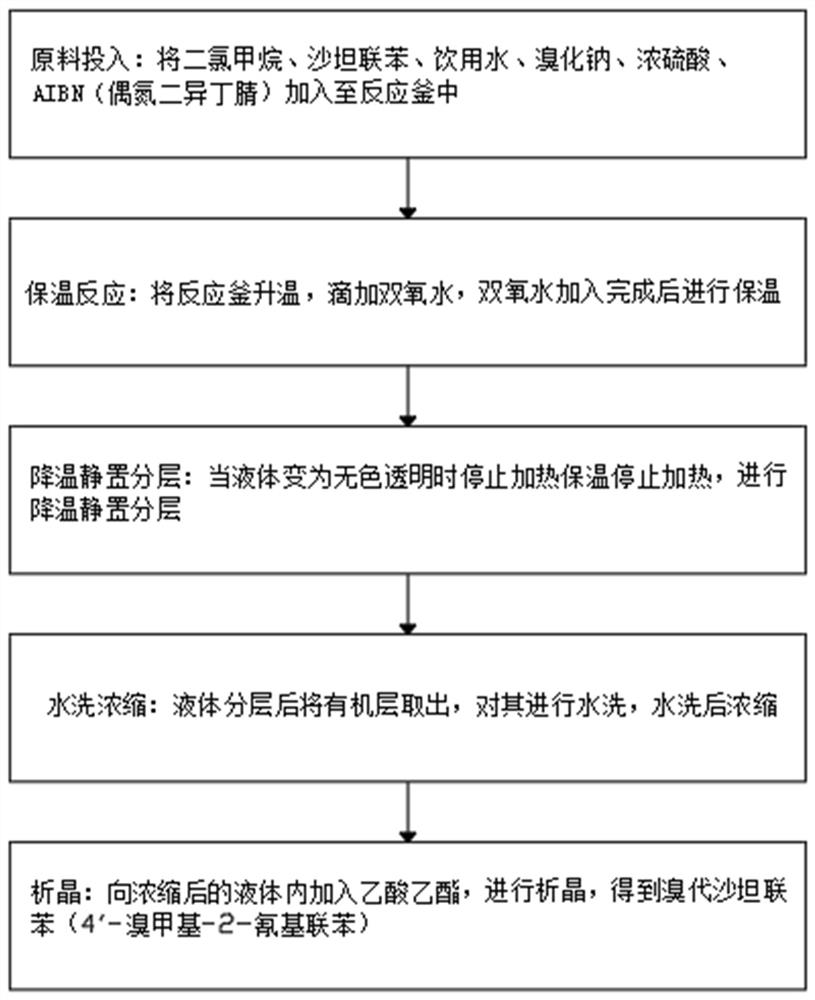
[0025] see figure 1 , the present invention provides the following technical solutions:
[0026] A kind of sodium bromide applies mechanically experimental method, comprises the steps:
[0027] Step 1, raw material input: add dichloromethane, sartan biphenyl, drinking water, sodium bromide, concentrated sulfuric acid, AIBN (azobisisobutyronitrile) into the reaction kettle;
[0028] Step 2, heat preservation reaction: heat up the reactor, add hydrogen peroxide dropwise, and keep heat after the hydrogen peroxide is added;
[0029] Step 3, cooling and standing for stratification: when the liquid becomes colorless and transparent, stop heating and heat preservation, stop heating, and carry out cooling and standing for stratification;
[0030] Step 4, washing and concentrating: taking out the organic layer after the liquid is separated, washing it with water, and concentrating after washing;
[0031] Step 5, crystallization: add ethyl acetate to the concentrated liquid for cryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com