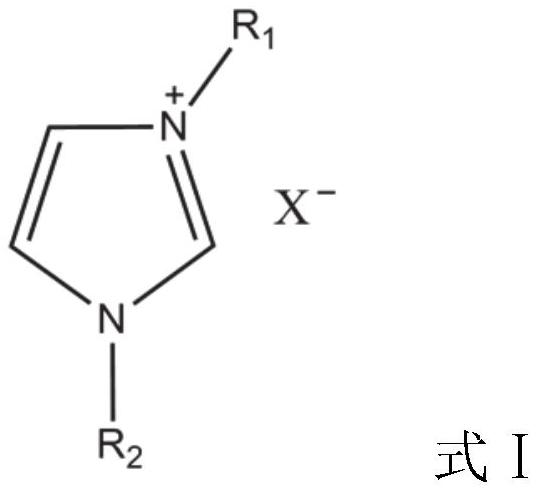
Method for preparing 5-hydroxymethylfurfural
A technology of hydroxymethylfurfural and acidic catalysts, which is applied in organic chemistry and other fields, can solve the problems of few preparation methods of HMF, and achieve the effects of large-scale industrialization, good stability, and easy scale-up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Weigh 100 g of 1-propyl-3-methylimidazolium chloride, 20 g of fructose, 0.01 g of 98% concentrated sulfuric acid, mix well, heat to 50 ° C for 6 h, and depressurize to 1000 Pa during the reaction (i.e. the system pressure is 1000Pa), remove the water content generated by the reaction, after the reaction is completed, the HMF concentration in the solution is detected by HPLC. The yield reaches 98%, and the selectivity is 99%. The vacuum was evacuated to 1000 Pa, the temperature was raised to 115° C., and 13.2 g of white solid crystals were obtained by distillation, wherein the HMF content was 99.5%.
Embodiment 2
[0068] Weigh 100g of 1-pentyl-3-butylimidazolium hexafluorophosphate, 20g of sucrose, and 0.5g of chromium chloride, mix well, heat to 80°C for 2 hours, and depressurize to 5000Pa during the reaction (ie, the system The pressure is 5000Pa), remove the moisture content generated by the reaction, after the reaction is completed, the HMF yield in the solution detected by HPLC reaches 96%, and the selectivity is 98%. The vacuum was evacuated to 100 Pa, the temperature was raised to 113° C., and 13.0 g of white solid crystals were obtained by distillation, wherein the HMF content was 99.2%.
Embodiment 3
[0070] Weigh 100 g of 1-propyl-3-dodecylimidazole trifluoroacetate, 20 g of glucose, and 0.5 g of β molecular sieve, mix well, heat to 70 ° C for 2 h, and depressurize to 500 Pa during the reaction (ie The system pressure is 500 Pa), and the moisture generated by the reaction is removed. After the reaction is completed, the HMF yield in the solution detected by HPLC reaches 96%, and the selectivity is 96%. The vacuum was evacuated to 500 Pa, the temperature was raised to 115° C., and 13.1 g of internal white solid crystals were obtained by distillation, wherein the HMF content was 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com