High-yield vanillin synthesis process
A synthesis process and vanillin technology are applied in the field of vanillin synthesis technology and high-yield vanillin synthesis technology, which can solve problems such as unsatisfactory yield, difficulty in separation and purification, and achieve less by-products and strong reference Significance, effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
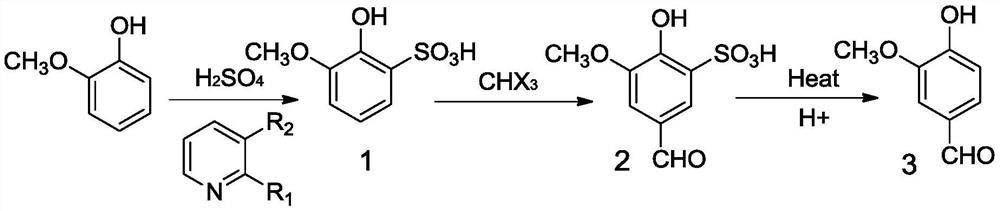
[0046]In a three-mouth flask with a thermometer, 17.5 g of pyridine, 300 g of tetrahydrofuran was added, and 20 g was slowly added 20 g from 98% concentrated sulfuric acid to the reaction system at 0 ° C to obtain a pyridine complex, and the elemental analysis is as follows: C37.49%, H 3.78%, N 8.74%, O 29.97%, S 20.02%. Then, 50 g of a cew pony phenol was added dropwise, and then the reaction was added after 15 min and then added a small amount of water and purified to give the product 1. 50 g of a compound 1 was dissolved in a solution of 120 g of ethanol, and 171.5 g 40% sodium hydroxide solution and 71.6 g of trichloromethane were added thereto, and the heated to 70 ° C, and the reaction was reacted under this condition, and purified to obtain Compound 2. Element analysis is as follows: C 41.38%, H 3.47%, O 41.34%, S13.81%, and the nuclear magnetic results are as follows:1H NMR (600MHz, CDCL3: Δ2.0 (1H), 3.83 (3H), 5.35 (1H), 7.27 (1H), 7.73 (1H), 9.88 (1H). 40 g of 50 g of Comp...
Embodiment 2
[0048]In a three-mouth flask with a thermometer, 41.2 g of 2-methylpyridine, 300 g of toluene was added, and 112 g of 70% concentrated sulfuric acid was slowly added dropwise to the reaction system at 0 ° C ice bath conditions to obtain a pyrix sulfonic acid complex. Elemental analysis is as follows: C41.37%, H 4.63%, N 8.04%, O 27.55%, S 18.41%. Then, 50 g of cewulphenol was added dropwise, and then the reaction was added after 50 min and then added a small amount of water and purified to obtain Compound 1. 50 g of compound 1 was dissolved in a 120 g of aqueous methanol solution, and 220.5 g 40% sodium hydroxide solution was added thereto and 114.6 g of trichloromethane, heated to 50 ° C, and reacted under this condition for 12 h, separated purification to obtain Compound 2. 40 g of 50 g of Compound 2 was dissolved in 200 g of water, stirred with a small amount of 30% dilute sulfuric acid to pH to 2.5, 50 min, and a gray oily liquid was precipitated, and a certain amount of acetate...
Embodiment 3
[0050]In a three-mouth flask with a thermometer, 51.5 g of 3-methylpyridine, 300 g of tetrahydrofuran was added. Under -10 ° C, 79.5 g 50% concentrated sulfuric acid was slowly added dropwise to obtain a pyrix sulfonate complex. Elemental analysis is as follows: C41.37%, H 4.63%, N 8.04%, O 27.55%, S 18.41%. Then, 50 g of a cew pony phenol was added dropwise, and after the addition was added, it was added after 40 min and then added a small amount of water, and purified to obtain Compound 1. 50 g of compound 1 was dissolved in 120 g of an aqueous solution of ethanol, and 196 g 40% sodium hydroxide solution and 86 g of chloroform were added thereto, and heated to 60 ° C, and the reaction was reacted under this condition, and purified to obtain Compound 2. 40 g of 50 g of Compound 2 was dissolved in 200 g of water, and stirred at a small amount of 30% dilute sulfate to pH to 1.5, 30 min, and a gray oily liquid was precipitated, and a certain amount of tare acetate was added to the oil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com