Preparation method of silicon dioxide
A silicon dioxide and hydrogen peroxide technology, applied in chemical instruments and methods, inorganic chemistry, silicon compounds, etc., can solve the problem of low dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation method of silicon dioxide of the present invention comprises the aforementioned step (1), step (2) and step (3).
[0018] [step 1)]
[0019] This step (1) is to provide a sodium silicate aqueous solution.
[0020] This sodium silicate aqueous solution contains sodium silicate and water.
[0021] Preferably, the sodium silicate aqueous solution is prepared by mixing sodium silicate and water and stirring. In a specific example of the present invention, the sodium silicate aqueous solution is prepared by mixing sodium silicate and water, heating and stirring.
[0022] Preferably, the above heating and stirring temperature ranges from 80°C to 95°C. More preferably, the temperature range is 90°C to 95°C.
[0023] [step (2)]
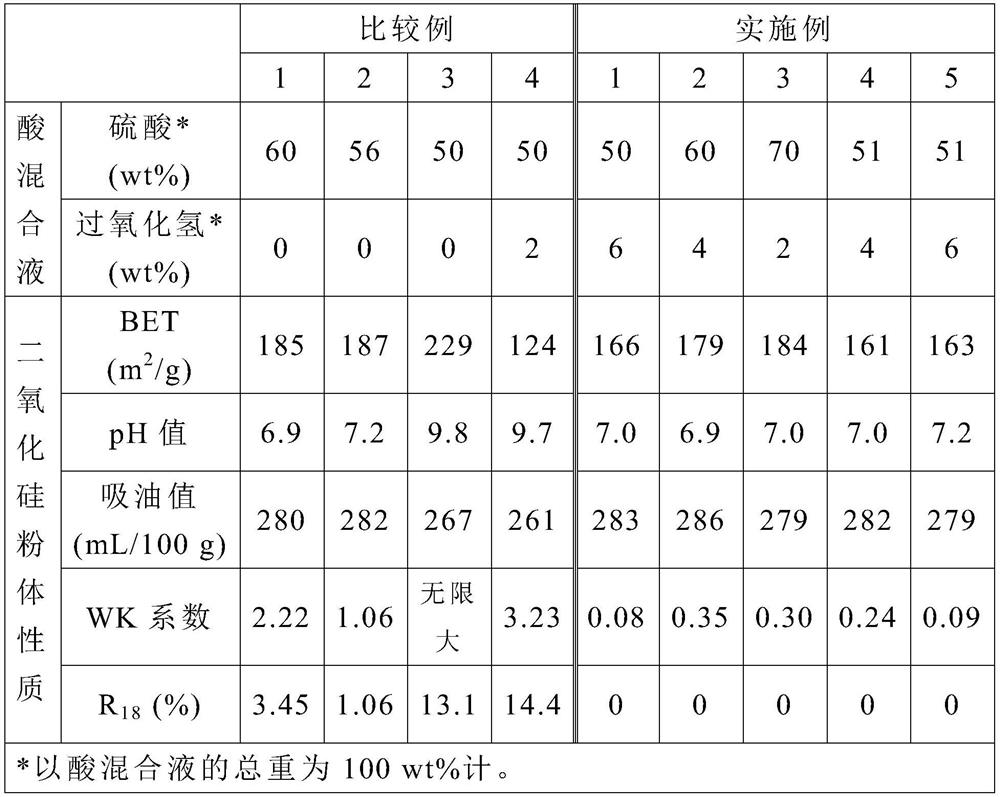
[0024] The step (2) is to react the sodium silicate aqueous solution with an acid mixture under an environment with a pH value greater than 7 to obtain a reaction solution, wherein the acid mixture contains sulfuric acid and hydrog...
Embodiment 1
[0041] The silica of embodiment 1 is made by following steps:
[0042] Step (1): 125g aqueous sodium silicate (Na 2 O·xSiO 2 ·nH 2 O; Na 2 O:9.23wt%, SiO 2 : 28.23wt%) and 3076g water were stirred and mixed (300rpm rotating speed) and the temperature was raised to 92°C to obtain a sodium silicate aqueous solution.
[0043] Step (2): Mix 372.5 g of acid mixture (containing sulfuric acid, hydrogen peroxide and water) with the aqueous sodium silicate solution obtained in step (1), and stir for 30 minutes to react to obtain a reaction liquid. In this step, based on the total weight of the acid mixture as 100wt%, the weight of sulfuric acid is 50wt%, the weight of hydrogen peroxide is 6wt%, and the temperature during the reaction is controlled at 92°C, and the pH is controlled at 7.8-8.2 During this period, the acid mixture was fed in 31 minutes, and the sodium silicate aqueous solution was fed in 24 minutes.
[0044] Step (3): Add 32.5g of acid mixture to reduce the pH value o...
Embodiment 2
[0047] The preparation method of embodiment 2 is similar to embodiment 1, and its difference is that step (2) and step (3) of embodiment 2 are as follows:
[0048] Step (2): Mix 313.5 g of acid mixture (containing sulfuric acid and hydrogen peroxide) with the aqueous sodium silicate solution obtained in step (1), and stir for 30 minutes to react to obtain a reaction liquid. In this step, based on the total weight of the acid mixture as 100wt%, the weight of sulfuric acid is 60wt%, and the weight of hydrogen peroxide is 4wt%, and the temperature during the reaction is controlled at 89 ° C, and the pH is controlled at 7.8 to 8.2 During this period, the acid mixture was fed in 30 minutes, and the sodium silicate aqueous solution was fed in 23 minutes.
[0049] Step (3): add 28.0g of acid mixed solution to reduce the pH value of the reaction solution obtained in step (2) to 4, and then react the reaction solution with a pH value of 4 at 91° C. for 1 hour to obtain two Silica solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Oil absorption value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com