A gene-edited microneedle for the treatment of inflammatory skin diseases and its application
An inflammatory skin disease, gene editing technology, applied in skin diseases, microneedling, gene therapy, etc., can solve the problem of difficult to reach the epidermis and dermis, and achieve the effect of improving the efficiency of gene editing and improving the inflammatory environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of Gene Editing Microneedle Patches
[0024] The present embodiment includes the following raw materials by mass percentage:
[0025] Needle tip matrix: collagen tripeptide 10%, sodium hyaluronate (molecular weight 34kDa) 10%, and the rest is water.
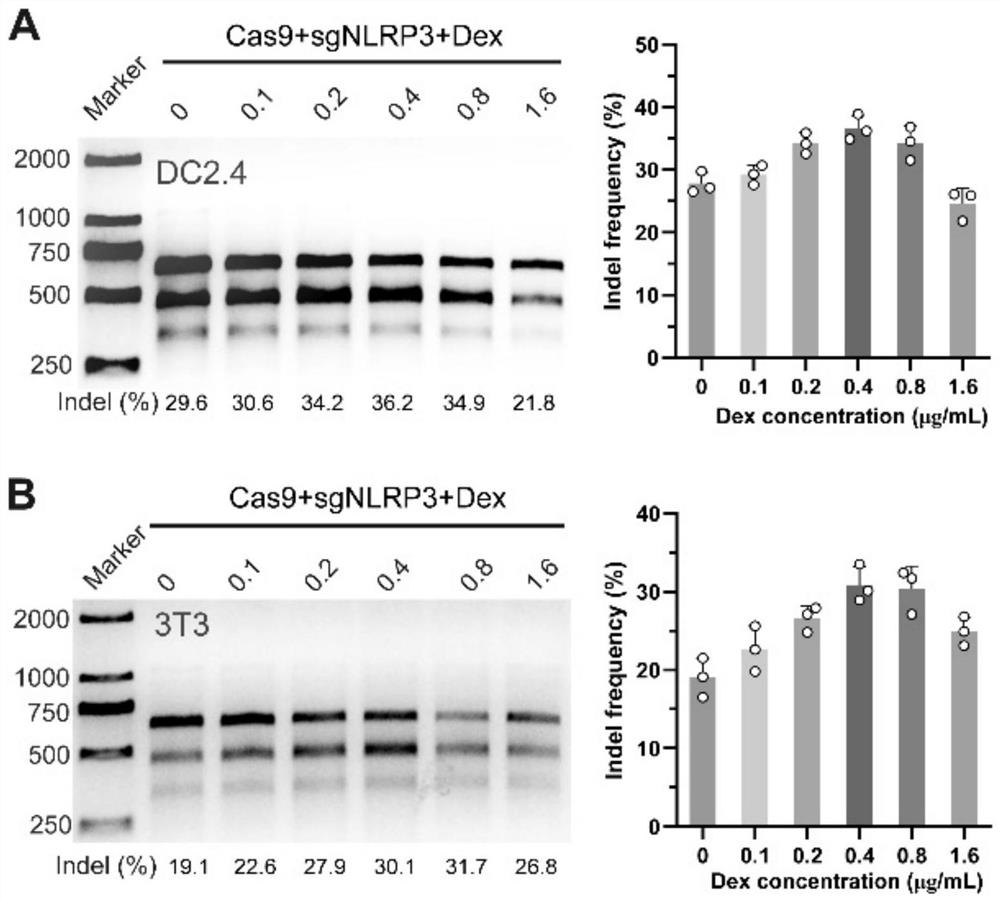
[0026] In addition, each 10 mL of sodium hyaluronate + collagen tripeptide solution in the microneedle tip matrix contained 60 μg of dexamethasone, 20 ug of Cas9 protein and 5 ug of sgRNA.
[0027] Base matrix: collagen tripeptide 10%, sodium hyaluronate (molecular weight 34kDa) 10%, and the rest is water.
[0028] The preparation method of the present embodiment:
[0029] 1. According to the formula ratio, disperse 1g of sodium hyaluronate (molecular weight 34kDa) and 1g of collagen tripeptide in 10mL of water, stir until fully dissolved, and add the dexamethasone nanocomposite encapsulated by the degradable polymer carrier in the prescribed proportion PLGA / Dex and a degradable cationic carrier e...
Embodiment 2
[0031] Example 2: Characterization of Gene Editing Microneedle Patches
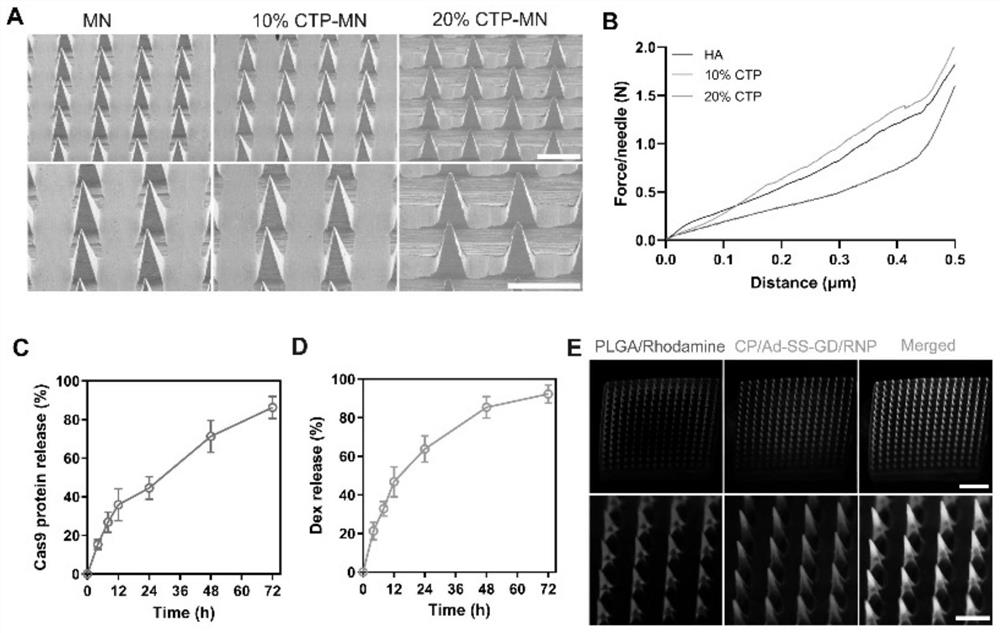
[0032] The specific operation method is as follows: the morphology of microneedles with different percentages of collagen tripeptide was observed by scanning electron microscope. The microneedles containing different percentages of collagen tripeptide were placed on the texture analyzer, the needle tip was upward, and the probe of the texture analyzer moved downward at a speed of 1 mm / s, and the mechanical strength was reflected by measuring the curve of displacement and force.
[0033] In order to investigate the dissolution of microneedles in the skin, the double-loaded microneedle patch was applied to the back skin of mice for 0, 1, 3 and 5 min, and then removed, and then the remaining microneedle patch was taken out and photographed with a scanning electron microscope.
[0034] To evaluate the in vitro drug release behavior of the microneedles, the double-loaded microneedle patch was first immersed in...
Embodiment 3
[0036] Example 3: Drug distribution after the gene-edited microneedle patch penetrates the skin
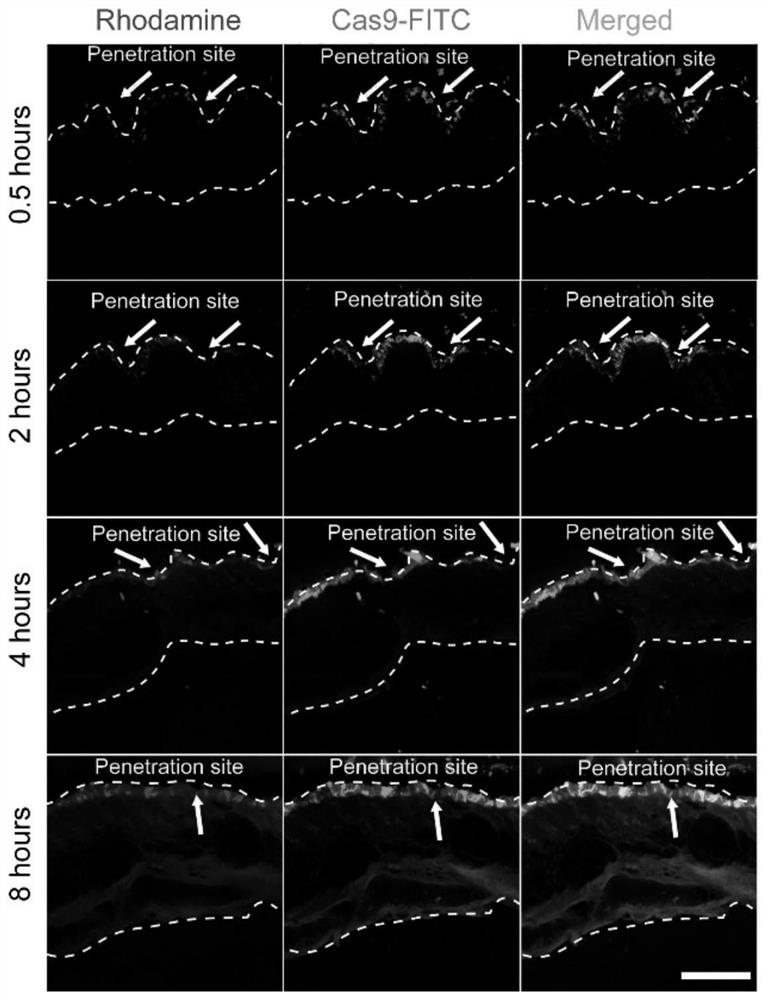
[0037] In order to study the degradation behavior of microneedles in vivo, a double-loaded microneedle patch was prepared with PLGA / Rhodamine B and CP / Ad-SS-GD / Cas9protein-FITC, and then the double-loaded microneedle patch was applied to the back of mice At different times of the skin, the back skin tissue was isolated for cryo-embedding, and then the tissue was placed in cryosections for sectioning, and the fluorescence distribution was observed under a confocal laser microscope.
[0038] Experimental results: as figure 2 As shown, the microneedle patch can successfully penetrate the mouse skin with a depth of about 300 μm. After piercing the mouse skin, the microneedle tip part can quickly absorb the liquid in the skin tissue and dissolve quickly, while rhodamine and Cas9-FITC Large amounts are released and distributed in the epidermis and dermis. Fluorescence images at diffe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com