Preparation and application of milk exosome loaded icariin nano preparation
A technology of icariin and nano preparations, applied in nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of toxicity and high cost, achieve low toxicity, low cost, and improve efficacy and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Extraction method of bovine milk exosomes
[0046] 1) Centrifuge fresh milk at 13000g in a 60mL centrifuge bottle for 30min at 4°C, and take the middle whey;
[0047] 2) 600-800 mesh vacuum pump filtration;
[0048] 3) Centrifuge at 100,000g for 2 hours at 4°C, and take the middle whey;
[0049] 4) 1000-2000 mesh vacuum pump filtration;
[0050] 5) Centrifuge at 13500g for 1.5h at 4°C, leave 1-2ml of the bottom, and collect in a 50ml EP tube;
[0051] 6) Centrifuge at 100,000 g for 2 hours at 4°C, discard the supernatant, and separate the milk exosome suspension;
[0052] 7) Mixing icariin and exosome suspension at a volume ratio of 1:9 at room temperature; freeze-drying to obtain the exosome-loaded icariin nanomedicine.
Embodiment 2
[0054] Identification of bovine milk exosomes
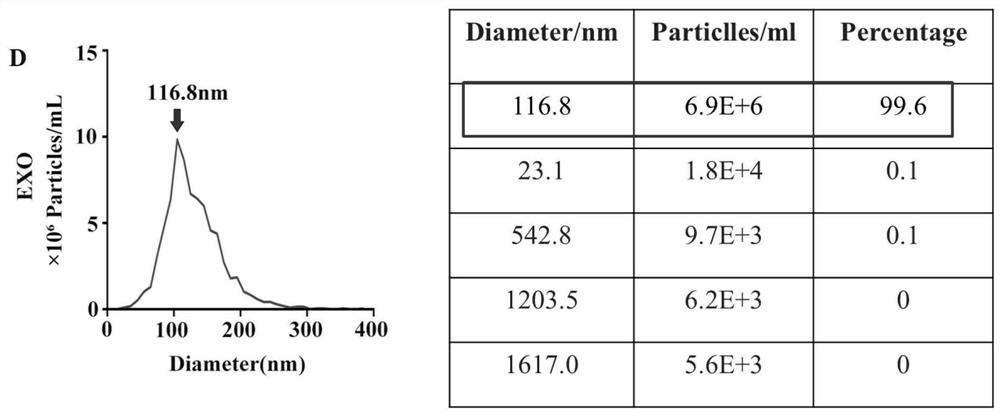
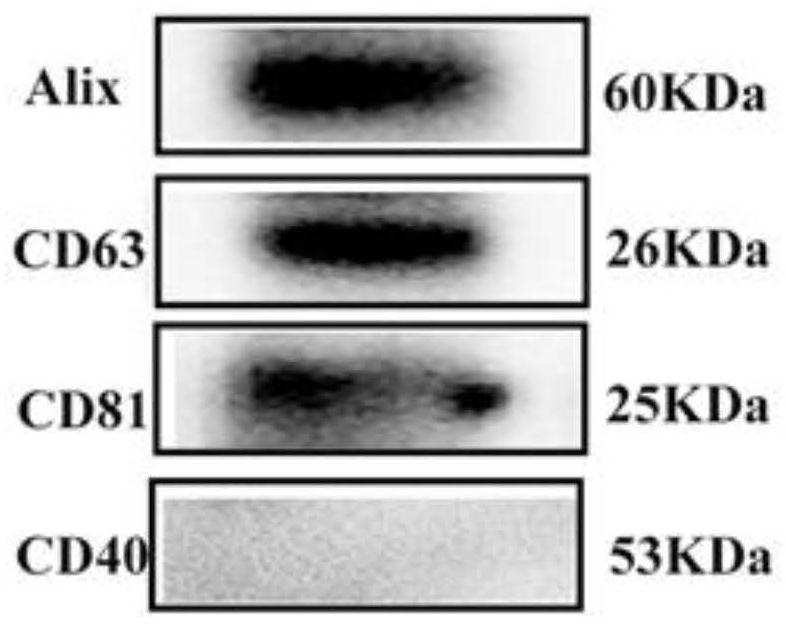
[0055] The size, structure and morphology of exosomes were observed by nanoparticle tracking analysis and transmission electron microscopy. The protein expression levels of exosome surface marker factors CD63, ALIX, CD81 and microcapsule surface marker CD40 were detected by Western Blot. Exosomes were labeled with PKH67 fluorescent staining, and the uptake of exosomes by MC3T3-E1 was detected.
Embodiment 3
[0057] Synthesis of Exosome-loaded Icariin Nanomedicine
[0058] Mix icariin and exosome suspension at a volume ratio of 1:9 at room temperature. After freeze-drying, the exosome-loaded icariin nanomedicine was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com