Primary mammary epithelial cell culture medium, culture method and application
A breast epithelial cell and primary cell technology, applied in the field of cell efficacy evaluation and screening of drugs, can solve the problems of high cost, necrosis, impact on response results, etc., and achieve a high success rate, high efficiency, and controllable culture cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Isolation of human primary mammary epithelial cells and optimization of primary mammary epithelial cell culture medium
[0060] (1) Isolation of human primary mammary epithelial cells
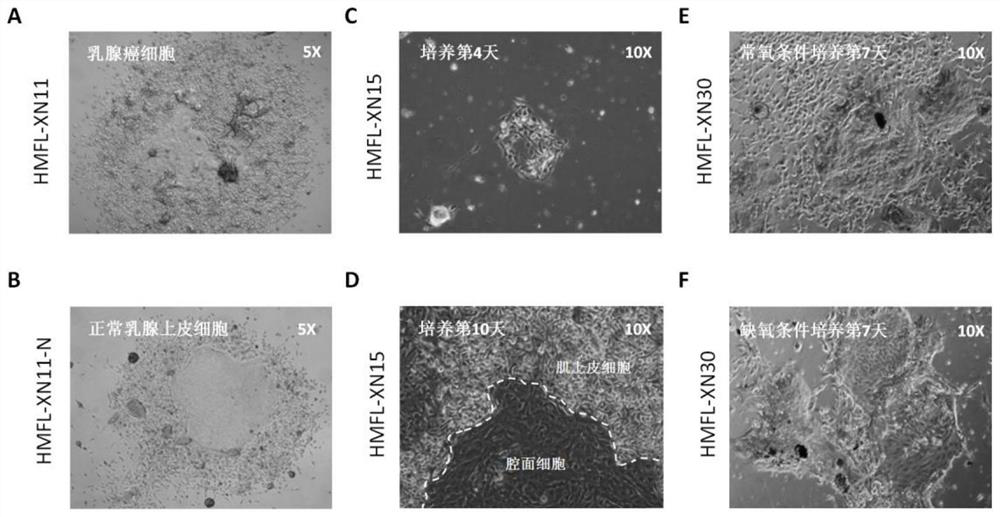
[0061]Breast cancer tissue samples were obtained from five breast cancer patients who had been explained and consented to surgically resected cancer tissue samples, which were HMFL-XN1, HMFL-XN3, HMFL-XN4, HMFL-XN6, and HMFL-XN8. One of the samples (HMFL-XN1) will be described below. The above tissue samples were collected within half an hour of the patient's surgical resection or biopsy. More specifically, in a sterile environment, tissue samples from non-necrotic sites were excised, with a volume of 0.5 cm 3 Above, it was placed in pre-cooled 20mL DMEM / F12 medium (manufactured by Corning Company), and the medium was contained in a 50mL plastic sterile centrifuge tube with a cover, and transported to the laboratory on ice; among them, the DMEM / F12 medium Contains 100U / mL penicillin a...
Embodiment 2
[0075] Culture of Human Primary Mammary Epithelial Cells
[0076] (1) Preparation of primary breast epithelial cell culture medium
[0077] First, prepare the basal medium according to the same method as step (2) of Example 1. Human Amphiregulin (manufactured by R&D Systems) was added to the basal medium at a final concentration of 20 ng / ml, EGF (manufactured by Peprotech) was added at a final concentration of 10 ng / ml, and B27 was added at a 1:50 dilution (Thermo Fisher SCIENTIFIC), human neurotonin 1 (manufactured by Peprotech) at a final concentration of 10 nM, FGF7 (manufactured by R&D Systems) at a final concentration of 10 ng / ml, and TGFβ1 inhibitor at a final concentration of 500 nM A8301 (manufactured by MCE), and P38 / MAPK inhibitor SB202190 (manufactured by MCE) were added at a final concentration of 500 nM to prepare a culture medium for primary mammary gland epithelial cells.
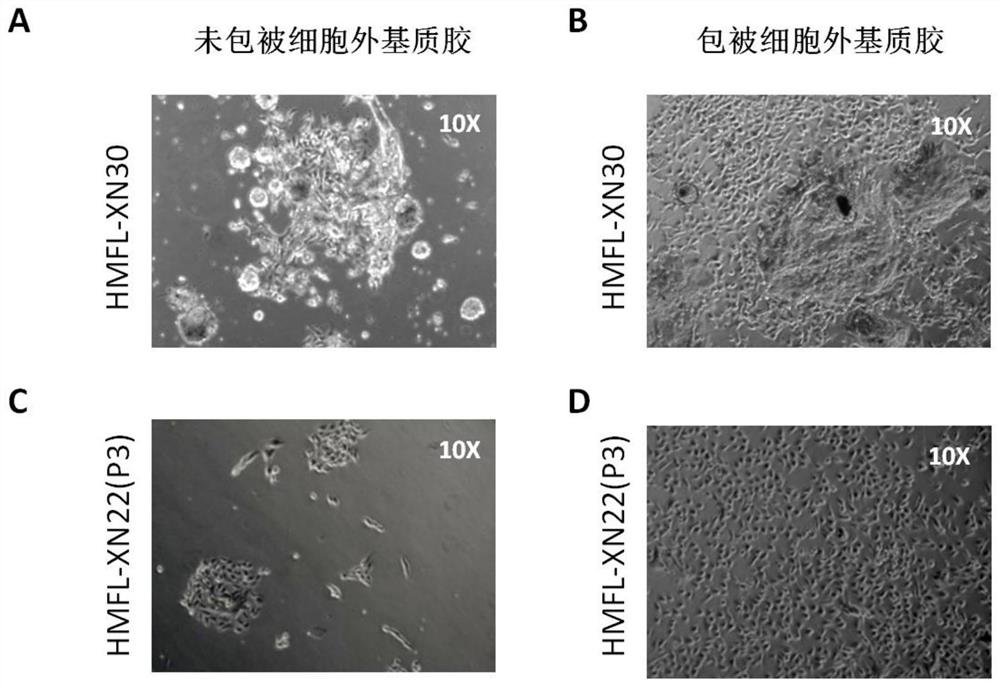
[0078] (2) Culture of primary breast cancer tumor cells derived from breast cancer tiss...
Embodiment 3
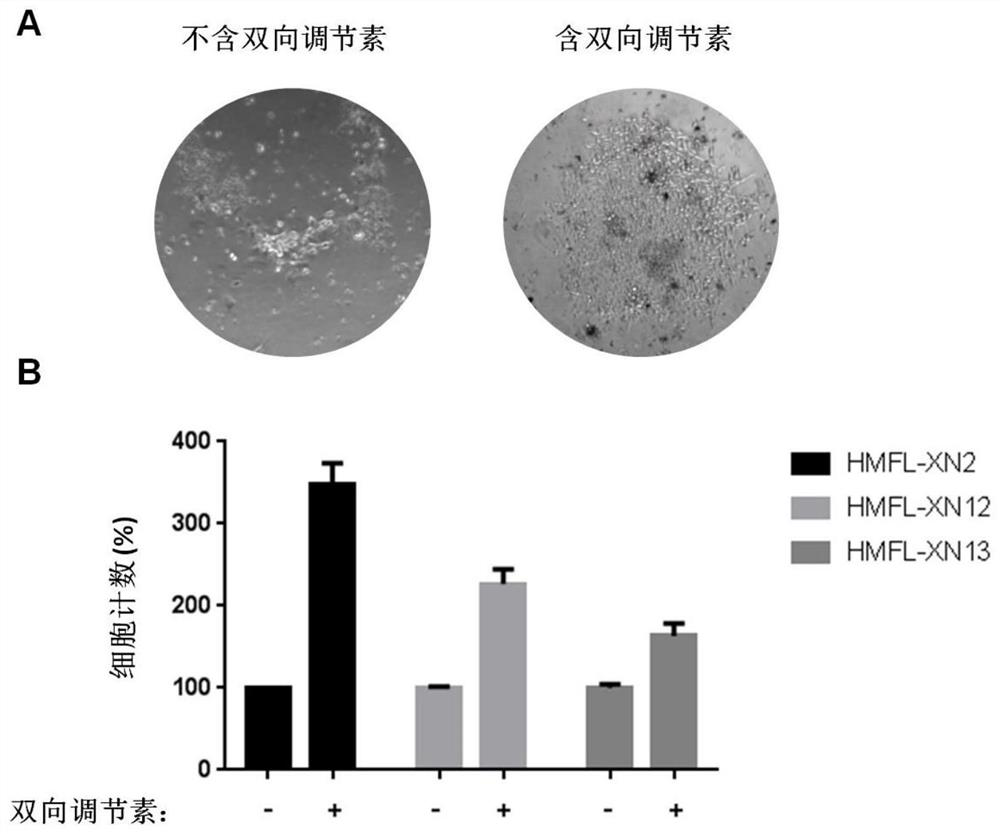
[0088] Effect of amphiregulin on the proliferation of primary breast cancer cells derived from breast cancer tissue
[0089] (1) The primary mammary gland epithelial cell culture medium was prepared by the same method as in step (1) of Example 2. In addition, amphiregulin was removed from the formula of primary mammary epithelial cell culture medium, and another primary medium without amphiregulin was prepared.
[0090] (2) Use the same method as step (1) of Example 1 to obtain primary breast cancer tumor cells (HMFL-XN2, HMFL-XN12) from two different cases of breast cancer patients and a case of breast cancer Primary breast cancer tumor cells (HMFL-XN13) from a patient biopsy.
[0091] Primary breast cancer tumor cells (HMFL-XN2) derived from surgically resected cancer tissue were inoculated at the same density (5×10 4 per well) were inoculated into 12-well plates coated with Matrigel (registered trademark) (manufactured by BD Biotechnology Co., Ltd.). The primary mammary ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com