N-heterocyclic carbene catalytic functionalized imine as novel 1, 4-dipole synthon and synthesis application thereof
A nitrogen-heterocyclic carbene and synthon technology, applied in the field of preparation of chiral nitrogen-heterocyclic compounds by 4+2 cyclization reaction, achieves the effects of reduced synthesis cost, good substrate universality, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The following preparation method was adopted to prepare (S)-IV01:
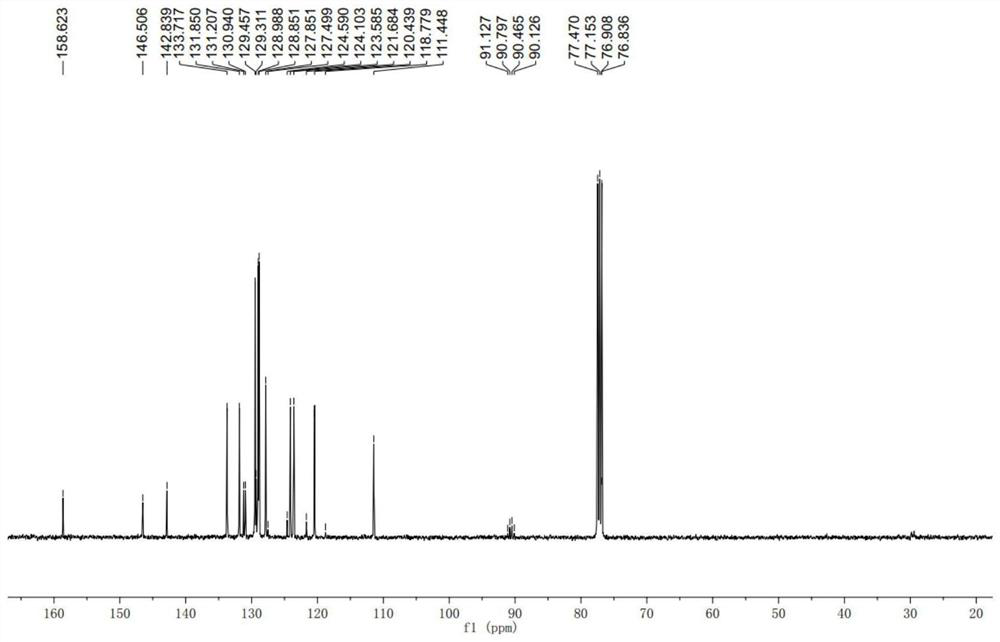
[0057] Add aldimine I (0.12mmol), NHC B (4.8mg, 20mol%), K 3 PO 4 (31.8mg, 0.15mmol), DQ (44.8mg, 0.11mmol), MS (100 mg), then 1 mL of dichloromethane was added to the mixture as a solvent, and finally 1.0 times the amount of trifluoroacetophenone was added to the system. The reaction system was stirred at room temperature for 48 hours. After the completion of the reaction was monitored by TLC, the reaction mixture was separated and purified using a silica gel column. The eluent was ethyl acetate / petroleum ether / dichloromethane=1:8:1, and the product was a white solid. (S)-2,4-diphenyl-4-(trifluoromethyl)-4H-benzo[4,5]imidazo[1,2-c][1,3,5]oxadiazine: 85% yield, 93:7er.HPLC condition: Chiralpak IA (Hex / iPrOH=85 / 15, 1.0mL / min, t R (major)=17.7min,t R (minor) = 16.3min.
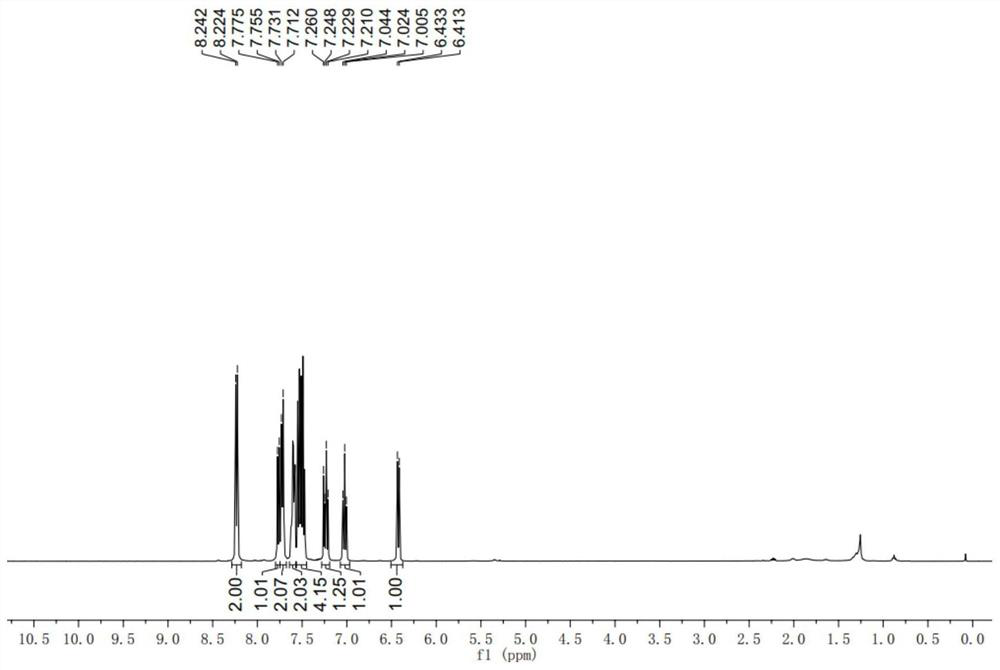
[0058] 1 H NMR (400MHz, CDCl 3 )δ8.24(d, J=7.2Hz, 2H), 7.77(d, J=7.6Hz, 1H), 7.73(d, J=7.6Hz, 2H), 7.63-7.59(m, 2H), 7.55- 7...
Embodiment 2
[0108] The following preparation method was adopted to prepare (R)-IV01:
[0109]Add aldimine I (0.12mmol), NHC C (4.8mg, 10mol%), Urea V (9.8mg, 20mol%), K 3 PO 4 (31.8mg, 0.15mmol), DQ (44.8mg, 0.11mmol), MS (100mg), then dichloromethane / n-hexane=(v / v=1:1, 0.1M) was added to the mixture as a solvent, and finally 1.0 times the amount of trifluoroacetophenone was added to the system. The reaction system was stirred at 0°C for 48 hours. After the completion of the reaction was monitored by TLC, the reaction mixture was separated and purified using a silica gel column. The eluent was petroleum ether / dichloromethane=1:6, and the product was a white solid.
[0110] (R)-2,4-diphenyl-4-(trifluoromethyl)-4H-benzo[4,5]imidazo[1,2-c][1,3,5]oxadiazine: 91% yield, 94:6er.HPLC condition: Chiralpak IA (Hex / iPrOH=85 / 15, 1.0mL / min, t R (major)=16.6min,t R (minor) = 17.9min.[α] D 25 (c 1.0, CHCl 3 ) = -53.3.
[0111] 1 H NMR (400MHz, CDCl 3 )δ8.24(d, J=7.2Hz, 2H), 7.77(d, J=7.6H...
Embodiment 3
[0163] Adopt the following preparation method to prepare VII01:
[0164] Add aldimine I (0.12mmol), NHC C (4.8mg, 10mol%), Urea V (9.8mg, 20mol%), K 3 PO 4 (31.8mg, 0.15mmol), DQ (44.8mg, 0.11mmol), MS (100mg) and N-Bn isatin 0.1mmol, then dichloromethane / n-hexane=(v / v=1:1, 0.1M) was added to the mixture as a solvent, and the reaction system was stirred at 0°C for 48 hours, After the completion of the reaction was monitored by TLC, the reaction mixture was separated and purified using a silica gel column, the eluent was ethyl acetate / petroleum ether / dichloromethane=1:4:1, and the product was a white solid.
[0165] (R)-1'-Benzyl-2-phenylspiro[benzo[4,5]imidazo[1,2-c][1,3,5]oxadiazine-4,3'-indole] -2'-Kone:96%yield,99:1er.[α] D 25 (c 1.0, CHCl 3 )=-64.8, HPLC condition: Chiralpak IA (Hex / iPrOH=70 / 30, 1.0mL / min, t R (major)=42.0min,t R (minor)=20.5min).
[0166] 1 H NMR (400MHz, CDCl 3)δ8.23(d,J=8.0Hz,2H),7.77(d,J=8.0Hz,1H),7.60-7.45(m,5H),7.32-7.18(m,7H),6.97(d,J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com