Application of trilobatin in preparation of medicine for treating acute liver injury
A technology of acute liver injury and trelobatin, applied in the field of pharmacy, can solve the problems of autophagy inducers to prevent and treat ALI, and achieve the effect of expanding the scope of functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
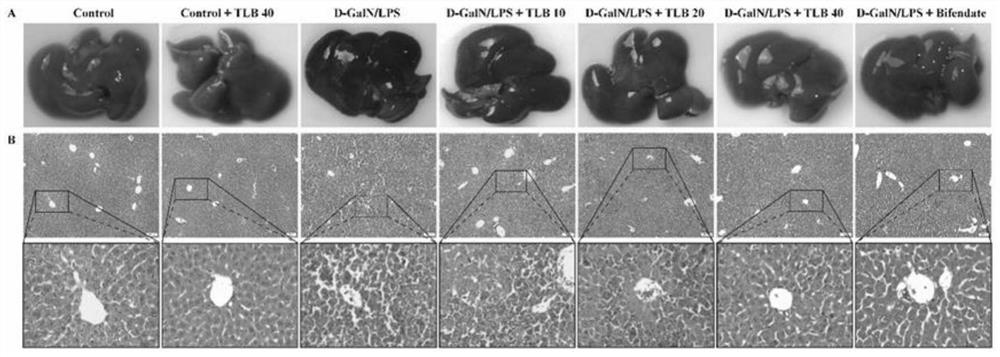
[0033]Trilobatin (TLB) protects mouse ali effect experiment
[0034]The reason for the establishment of a D-Galn / LPS: D-GalN / LPS-induced acute liver injury model is ideal, widely accepted and used as an animal model for exploration and evaluation of liver protector. Among them, GALN-induced animal liver tissue injury is similar to human acute severe hepatitis, while LPS can induce acute liver failure in GALN sensitizing mice. D-Galn / LPS can stimulate liver macrophages produce inflammation factors including tumornecrosis factor, TNF-α), interleukin, IL) -1β, IL-6, etc., resulting in inflammatory response, further damaging liver cells Even liver failure.
[0035]Effect of TLB on mouse Ali induced by D-Galn / LPS: TLB protected mouse Ali was observed using D-GalN / LPS-induced mouse Ali model. Male C57BL / 6 (body weight 18-22 g) mice were randomly divided into control group (CONTROL + TLB 40mg / kg, Model (D-Galn / LPS) + TLB 10mg / KG or TLB20 mg / kg or TLB 40 mg / kg treatment grou...
Embodiment 2
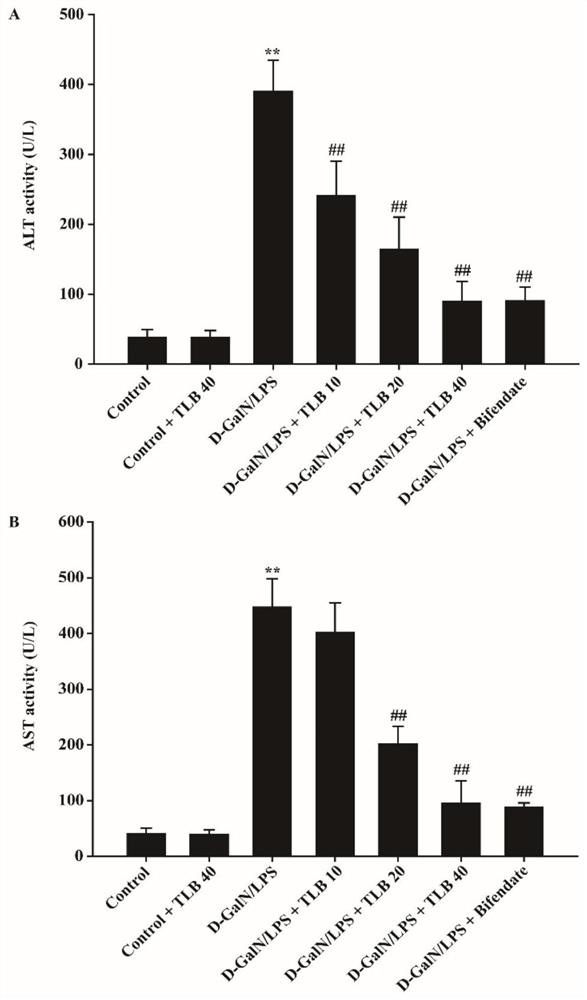
[0038]Effect of TLB on Ali Mouse Blood Clear Vallaenamase (Alt) and Valley Transuninaminase Activity (AST)
[0039]Male C57BL / 6 mice (body weight 18-22 g) were randomly divided into control group (CONTROL + TLB 40 mg / kg, model (d-galn / lps) control group, Model (D-Galn / LPS) + TLB 10mg / kg or TLB 20 mg / kg or TLB40 mg / kg treatment group, TLB dose group prophylactic administration was continuously irradiated for 7 days, and the Ali model was prepared by intraperitoneal injection D-Galn (700 mg / kg) / LPS (100 μg / kg). After 6 hours, serum, detect Alt and AST activity.
[0040]Such asfigure 2 As shown, it can be concluded by a graph, compared with normal group, the serum ALT activity in the model group is significantly increased (p<0.05); while TLB can reduce the activity of serum Alt in mice (P<0.05). From the B-graph, compared with the normal group, the serum AST activity in the model group is significantly increased (P<0.05); and TLB can reduce the activity of serum AST in mo...
Embodiment 3
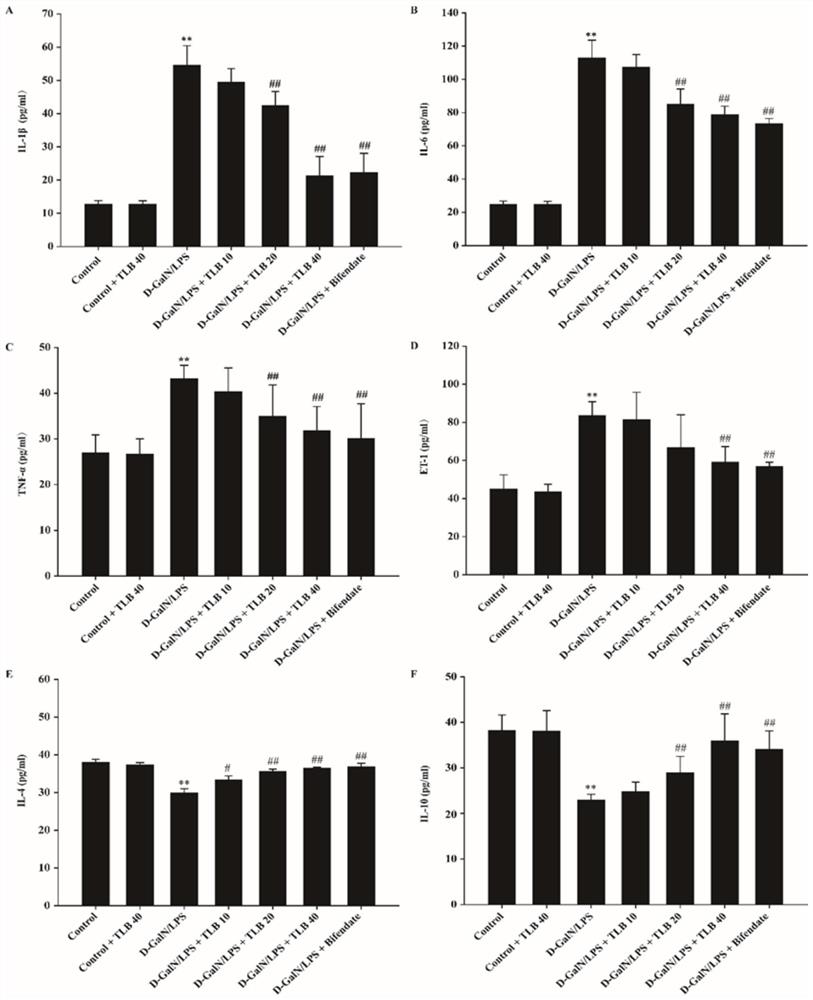
[0042]Effect of TLB on inflammation factors of Ali mice
[0043]Male C57BL / 6 (body weight 18-22 g) mice were randomly divided into control group (CONTROL + TLB 40mg / kg, Model (D-Galn / LPS) control group, Model + TLB 10 mg / kg or TLB 20 mg / kg Or TLB 40 mg / kg treatment group, TLB dosage group prophylactic administration continuously for 7 days, ALI model was prepared by intraperitoneal injection D-Galn (700 mg / kg) / lps (100 μg / kg). After 6 hours, serum and test the horizontal level.
[0044]Such asimage 3 As shown, A-D is shown, compared with normal group, the blood-proof of the model group mouse blood surgery factor TNF-α, IL-1β, IL-6, and ET-1 levels are significantly increased (P<0.05); TLB can reduce the level of serum TNF-α, IL-1β, IL-6, ET-1 in mouse (P<0.05). E-F Figure, compared with normal group, the level of blood-deficitanimoni IL-4 and IL-10 in the model group is significantly reduced (P<0.05); and TLB can raise the level of serum IL-4 and IL-10 in mice (P<0.05). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com