Hybrid antibacterial protein with strong bactericidal effect and application thereof
A bactericidal effect, antibacterial protein technology, applied in the direction of medical preparations with no active ingredients, medical preparations containing active ingredients, hybrid peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Construction, expression and purification of engineering bacteria of embodiment 1, AB469
[0039] 1. Construction of engineering bacteria of AB469
[0040] According to the codon preference of Escherichia coli, the nucleic acid sequence (SEQ ID NO: 2) capable of encoding AB469 was artificially designed and synthesized, namely ab469; an NcoI restriction endonuclease site (italic part) was added to its 5' end, and at the same time The nucleic acid sequence ct is introduced more, together with the g at the end of the restriction site, a codon encoding an alanine is formed; a HindIII restriction endonuclease site (italic part) is added at the 3' end. The sequence is as follows:
[0041] ccatgg ctatcctgaccaaagatggctttggcatcattcgcaacgaactgtttggcggcaaactggatcagacacaggtggatg ccattaattttattgttgaaaaagctaccgaatctgggttaagttatccggaagcggcctatctgttagcgacgatctatcatgaaacgggt ctgccgagcggttatcgtaccatgcagccaatcaaagaagccggtagtgataattacctccgctctaaaaaatattatccgtatatcggct atggctatgttcagctga...
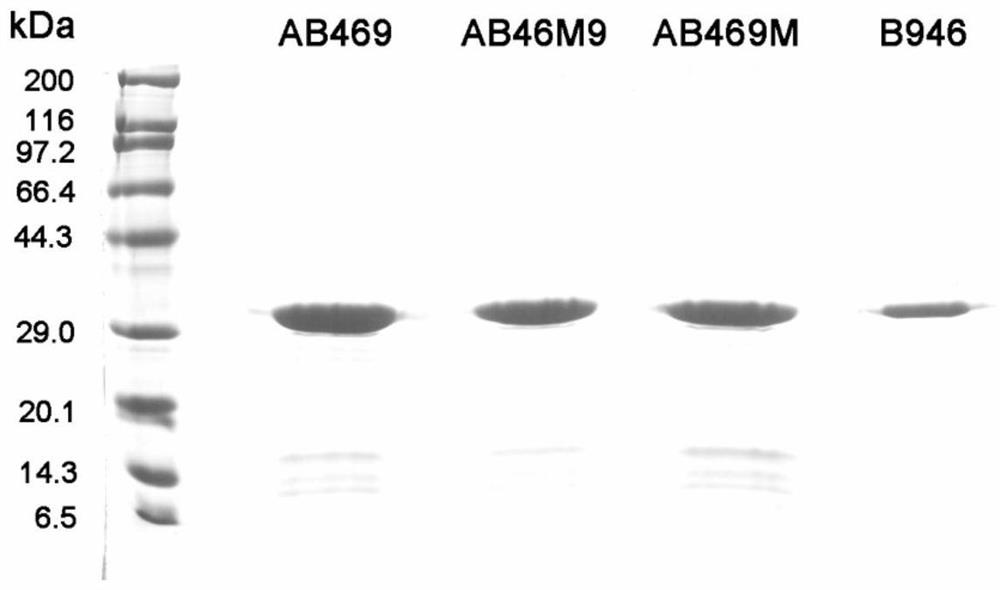
Embodiment 2、AB469
[0046] Construction, expression and purification of engineering bacteria of embodiment 2, AB469 mutants AB46M9, AB469M and B946
[0047] 1. Construction of mutant engineering bacteria
[0048] Mutant AB46M9 (SEQ ID NO: 3) is composed of a catalytic domain AB46M (SEQ ID NO: 10) and a binding domain SP10 (154- 236aa) (SEQ ID NO:11), with a flexible Linker in the middle.
[0049] The mutant AB469M (SEQ ID NO: 5) is composed of the catalytic domain AB46 (1-185aa) and the binding domain B9M (SEQ ID NO: 12) which has 82% similarity to the amino acid sequence of SP10 (154-236 aa), and the middle is A flexible Linker.
[0050] B946 (SEQ ID NO:7) is composed of the binding domain SP10 (154-236aa) and the catalytic domain AB46 (1-185aa), with a flexible Linker in the middle, and the positions of the catalytic domain and the binding domain are just opposite to those of AB469.
[0051] According to the codon preference of Escherichia coli, the nucleic acid sequences ab46m9 (SEQ ID NO 4...
Embodiment 3
[0053] Embodiment 3, turbidimetric method detects the bactericidal activity of AB469 to Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae
[0054] The overnight cultured Acinetobacter baumannii (ATCC 19606), Pseudomonas aeruginosa (ATCC 15442), and Klebsiella pneumoniae (ATCC 700603) were grown to mid-log phase (OD 600 About 0.5), centrifuge (5,000g, 5 minutes) to collect the bacteria. The cells were washed twice with 20mm PBS (pH 7.2), and then resuspended in PBS buffers containing different NaCl concentrations (0-600 mM). Add sample (final concentration 2 μg / mL) or PBS control, 10 μl 200 mm EDTA (final concentration 0.5 mm) and 50 μl bacterial suspension in 96-well plate, add PBS to total reaction system 200 μl, reaction temperature 37 ° C, in Read at a wavelength of 600nm (1 minute interval, 10 readings).
[0055] Turbidity test results see Figure 2-Figure 4 , AB469 can cause the OD value of the bacterial liquid to decrease by lysing the pathogenic ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com