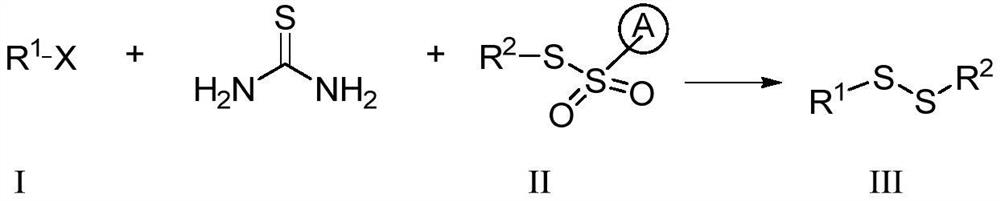
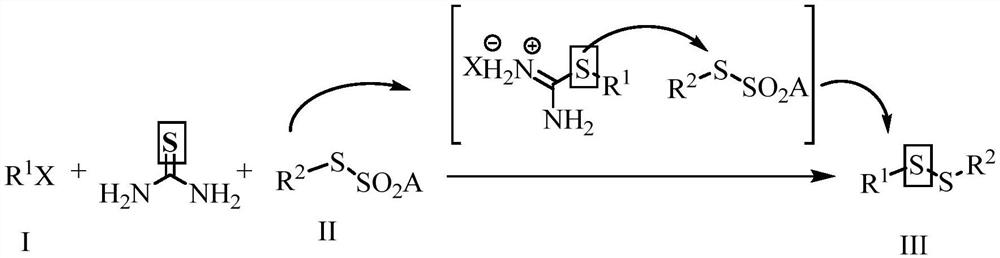
Preparation method of alkali-promoted asymmetric organic persulfide compound
A compound, asymmetric technology, used in the preparation of sulfides, organic chemistry, steroids, etc., can solve the problems of harsh reaction process requirements, complex systems, environmental pollution, etc. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0067] Synthesis of reference example 1 raw material formula (II) compound
[0068]
[0069] Among them, R 2 , Ring A is as defined in the present invention; Y is Cl or Br.
[0070] Add 4 mL of acetonitrile to a 10 mL two-neck Schlenk tube. Compound of formula (IV-1) (0.5 mmol) and compound of formula (V) (1.5 equivalents, 0.75 mmol) were added, and the reaction mixture was reacted at 100°C for 3 h, then turned to room temperature, and stirred overnight. Filtrate and concentrate the filtrate to obtain a crude product, which is separated by column chromatography to obtain a compound of formula (II).
reference example 2
[0071] Reference example 2 synthesis of raw material formula (II) compound
[0072]
[0073] Among them, R 2 , Ring A is as defined in the present invention.
[0074] Dissolve the compound of formula (IV-2) (1 equivalent) in acetonitrile, add the compound of formula (V) (1.5 equivalents), NBS (2.0 equivalents) and stir at room temperature, monitor the reaction by TLC, until the raw material disappears, add brine and ethyl acetate, the organic layer was separated, the aqueous phase was extracted with ethyl acetate, the combined organic layer was washed with brine, and washed with Na 2 SO 4 Drying and evaporation in vacuo afforded a crude product of the compound of formula (II-2), which was separated by column chromatography to give the compound of formula (II).
Embodiment 1
[0076]
[0077] In a dry 25 mL Schlenk reaction tube, add 0.5 mmol of 4-methoxyphenyl thiosulfonate, 1.0 mmol of thiourea, 1.0 mmol of benzyl bromide, 0.5 mmol of potassium carbonate, and 3 mL of toluene. Stir at 80°C for 24 hours. After the reaction, cool to room temperature, filter, concentrate the filtrate, and directly pass through a silica gel column (the volume ratio of ethyl acetate to petroleum ether is 1:50-1:3) to obtain the product with a yield of 92%.
[0078] 1 H NMR (400MHz, CDCl 3 )δ7.36–7.27(m,5H),7.18–7.15(m,2H),6.87–6.85(m,2H),3.81(s,3H),3.65(s,2H),3.57(s,2H) ; 13 C NMR (100MHz, CDCl 3 ) δ 159.0, 137.4, 130.5, 129.4, 129.2, 128.4, 127.4, 113.8, 55.2, 43.3, 42.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com