Method for electrochemical synthesis of indole compounds
A compound, electrochemical technology, applied in the field of electrochemical synthesis of indole compounds, can solve problems such as poor atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Put substrate (shown in formula 1-a) (0.3mmol, 82.0mg), potassium thiocyanate (0.3mmol, 29mg), tetramethylammonium iodide (0.3mmol, 60.3 mg), acetonitrile (5mL) and water (1mL), the platinum sheet electrode was used as both anode and cathode, and reacted under electric stirring (I=5mA) at 80°C. After the reaction was completed (TLC tracking detection), the residue obtained after rotary evaporation and concentration was passed through the chromatographic column with the ethyl acetate / petroleum ether system as the eluent to obtain the product N-Ts indole (shown in formula 1-b), the product The rate is 92%.
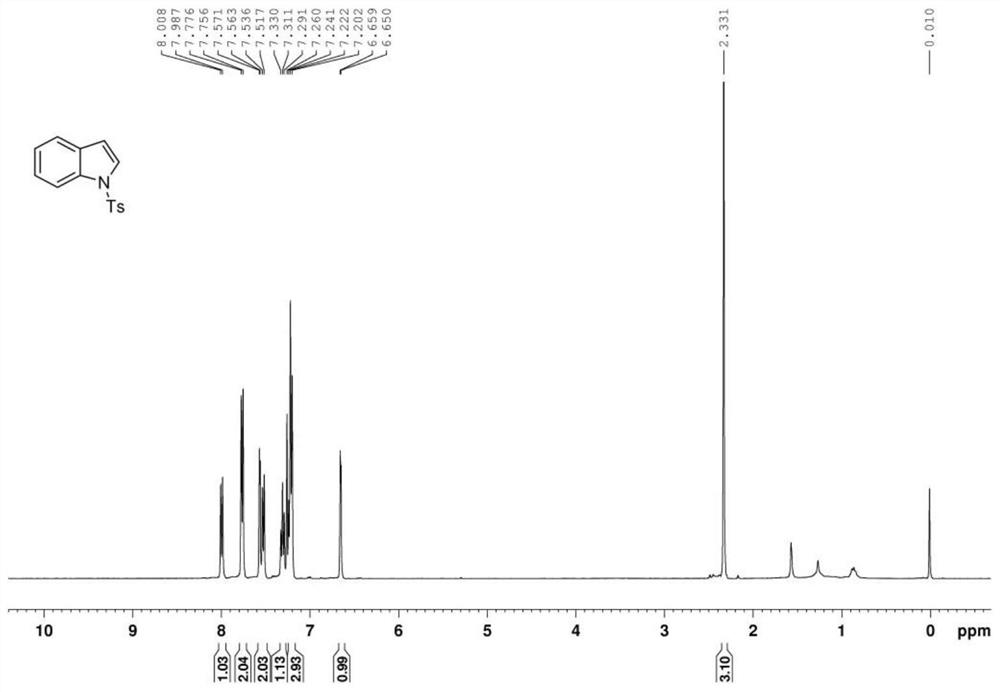
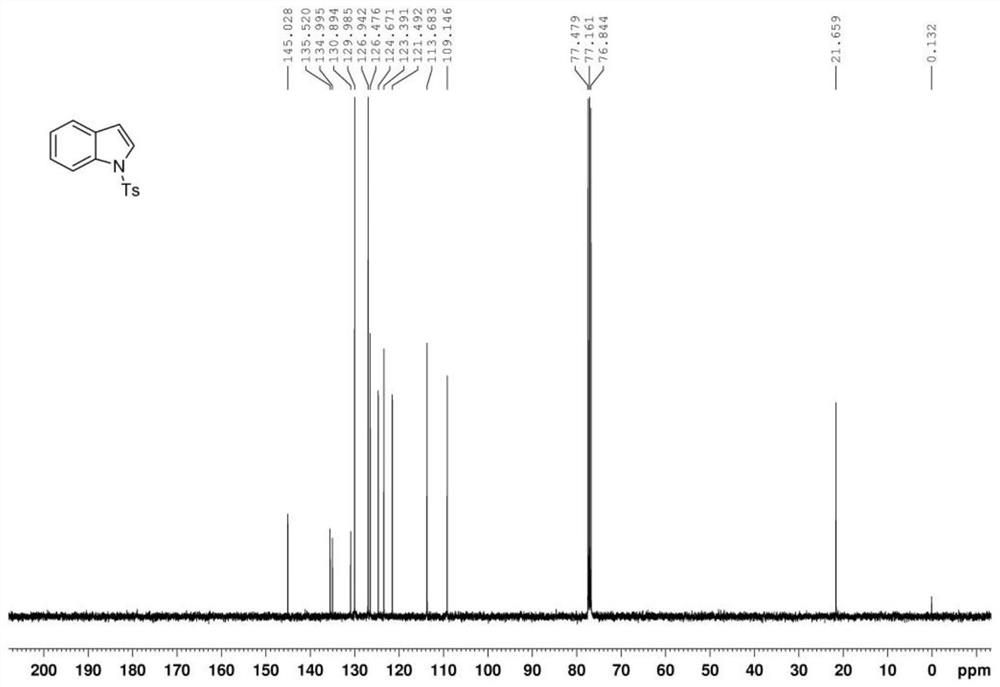
[0049] The indole product is analyzed by nuclear magnetic resonance spectrometer, the results can be found in Figure 1 ~ Figure 2 , figure 1 For the indole product that the embodiment of the present invention 1 provides 1 H NMR ( 1 H-NMR) spectrogram; figure 2 For the indole product that the embodiment of the present invention 1 provides 13 C NMR ( 13 C-NMR) ...
Embodiment 2
[0053] Put substrate (shown in formula 2-a) (0.3mmol, 89.5mg), potassium thiocyanate (0.3mmol, 29mg), tetramethylammonium iodide (0.3mmol, 60.3 mg), acetonitrile (5mL) and water (1mL), the platinum sheet electrode was used as both anode and cathode, and reacted under electric stirring (I=5mA) at 80°C. After the reaction was completed (TLC tracking detection), the residue obtained after rotary evaporation and concentration was used as an eluent through the chromatographic column to obtain the product 5-cyano N-Ts indole (formula 2-b) Shown), the productive rate is 66%.
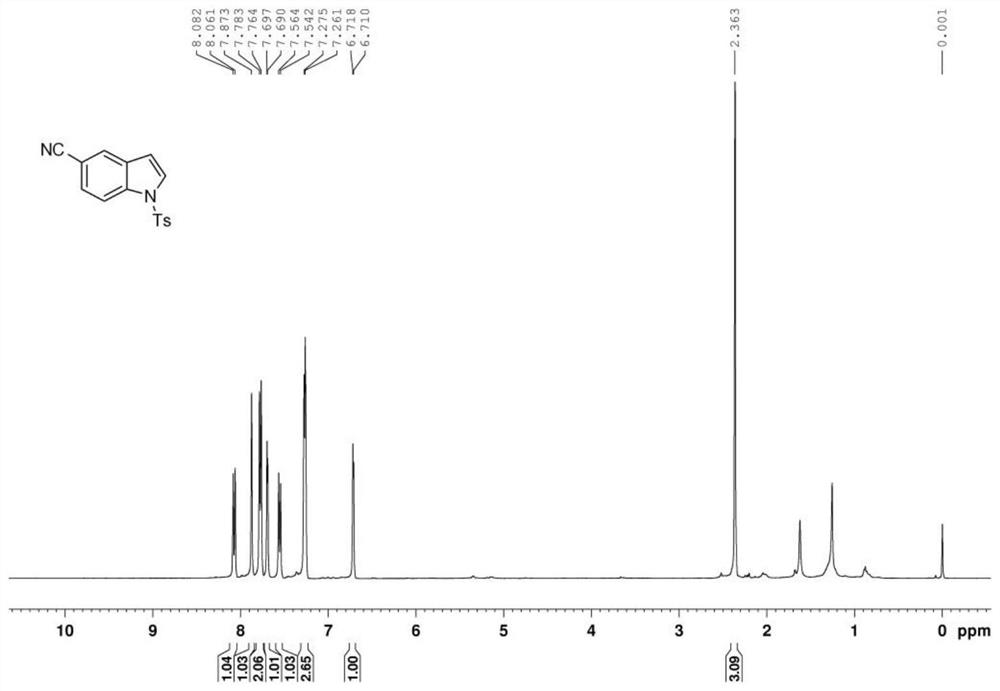
[0054] The indole product is analyzed by nuclear magnetic resonance spectrometer, the results can be found in Figure 3 ~ Figure 4 , image 3 For the indole product that the embodiment of the present invention 2 provides 1 H NMR ( 1 H-NMR) spectrogram; Figure 4 For the indole product that the embodiment of the present invention 2 provides 13 C NMR ( 13 C-NMR) spectrogram.
[0055] Described product is ...
Embodiment 3
[0058] Put substrate (shown in formula 3-a) (0.3mmol, 94.6mg), potassium thiocyanate (0.3mmol, 29mg), tetramethylammonium iodide (0.3mmol, 60.3 mg), acetonitrile (5mL) and water (1mL), the platinum sheet electrode was used as both anode and cathode, and reacted under electric stirring (I=5mA) at 80°C. After the reaction was completed (TLC tracking detection), the residue obtained after rotary evaporation and concentration was passed through the chromatographic column with ethyl acetate / petroleum ether system as the eluent to obtain the product 5-acetyl N-Ts indole (formula 3-b) Shown), the productive rate is 74%.
[0059] The indole product is analyzed by nuclear magnetic resonance spectrometer, the results can be found in Figure 5 ~ Figure 6 , Figure 5 For the indole product that the embodiment of the present invention 3 provides 1 H NMR ( 1 H-NMR) spectrogram; Figure 6 For the indole product that the embodiment of the present invention 3 provides 13 C NMR ( 13 C-NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com