Tetrodotoxin oral preparation, tetrodotoxin freeze-dried tablet and application of tetrodotoxin oral preparation
A technology for tetrodosine and oral preparations, applied in the field of oral preparations of tetrodosine, can solve the problems of not being able to show the antitumor effect of tetrodoxine, and that the drug concentration cannot reach the in vitro concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
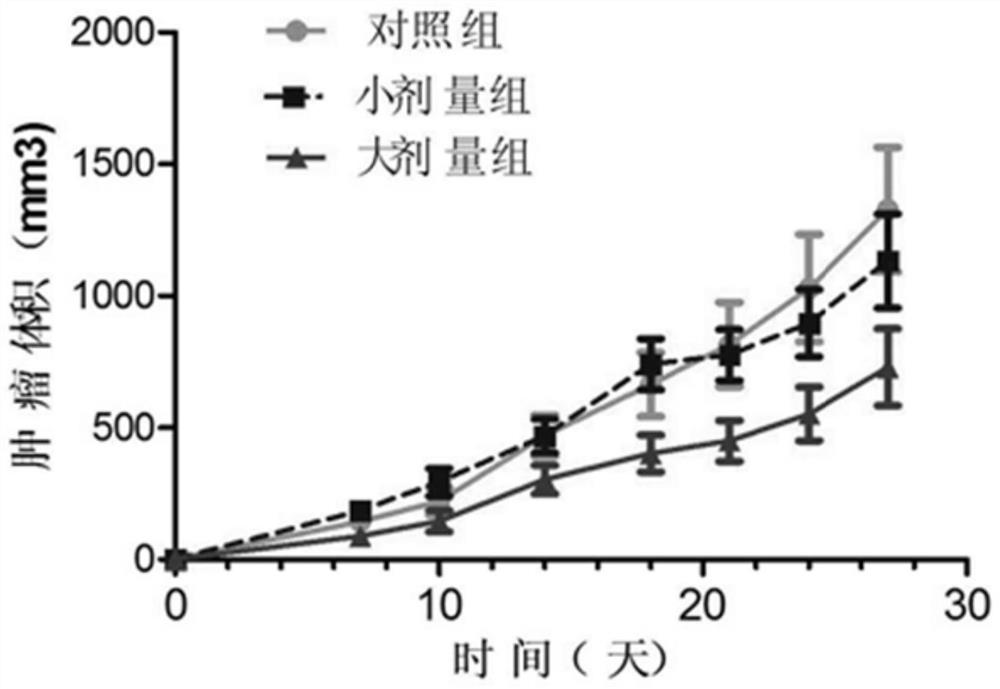
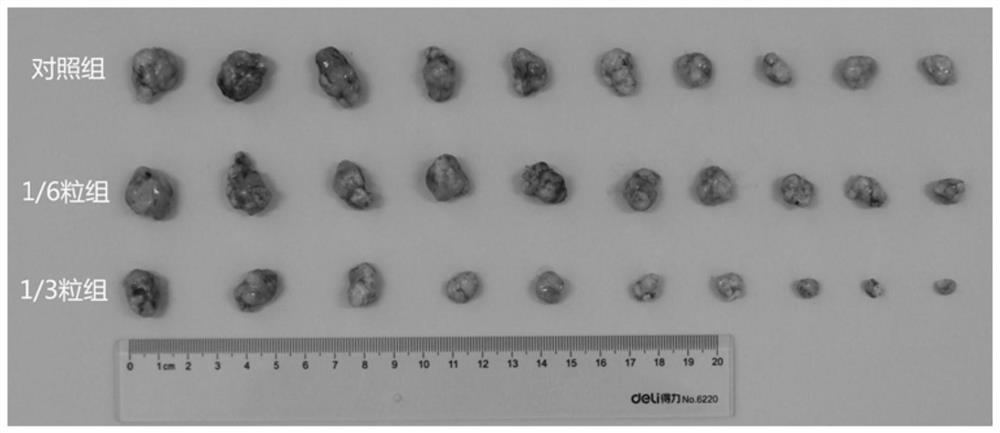
Image
Examples
Embodiment 1
[0068] Embodiment 1: Preparation of freeze-dried material of tetradosin and stabilizer lactose (abbreviated as tetradosin freeze-dried material)
[0069]
[0070] 1) Weigh 2200g of lactose, add 16kg of water, stir to dissolve.
[0071] 2) Weigh 42 g of citric acid and 58 g of sodium citrate, add 20 kg of water, stir to dissolve, and measure the pH to be 4.5 to 4.6; add 4.0 g of tetrodocine, stir to dissolve.
[0072] 3) Mix the above two solutions evenly, and the measured pH should be 4.5-4.9; add about 4kg of water to a total weight of about 43kg.
[0073] 4) The above-mentioned mixed solution is divided into containers, placed in a lyophilizer, and freeze-dried to obtain a tetradosin freeze-dried material.
Embodiment 2
[0074] Embodiment 2: preparation tetradoxine tablet
[0075]
[0076] 1) Take the above-mentioned prescription amount of tetradosin freeze-dried material, pass through a 24-mesh sieve, and set aside.
[0077] 2) Take the pregelatinized starch of the above prescription amount, pass through an 80-mesh sieve, add 8% starch slurry and use a 16-mesh sieve to make wet granules; after drying at 80°C, sieve the granules with a 24-mesh sieve for later use.
[0078] 3) Mix the above-mentioned two kinds of granules with the prescription amount of povidone K30 and magnesium stearate evenly, and compress into tablets (0.5g / tablet) to obtain 1000 tablets of tetrodosin tablets.
Embodiment 3
[0079] Embodiment 3: Preparation of Tetradosin Lyophilized Tablets
[0080]
[0081] 1) Take the citric acid, sodium citrate, lactose, mannitol, and sodium alginate in the above prescription amount, add them to the fully dissolved xanthan gum solution, mix well, and then add the above prescription amount of tetradol Simultaneously mix well to form a homogeneous solution.
[0082] 2) After degassing the above solution, pour it into the mold accurately; pre-freeze at -40°C to -170°C for 1 to 60 minutes, then transfer it to a lyophilizer. Freeze-drying at -30°C for 1 hour to 10 hours can give Tetradosin freeze-dried tablets.
[0083] In the above method, after the pre-freezing step, before transferring to the lyophilizer, an ice crystal incubation step can also be added, that is, the mold filled with the solution after pre-freezing is placed in a low-temperature environment of -5°C to -60°C for 0.5h to 15h.
[0084] The freeze-dried tablet prepared by this method disinteg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com