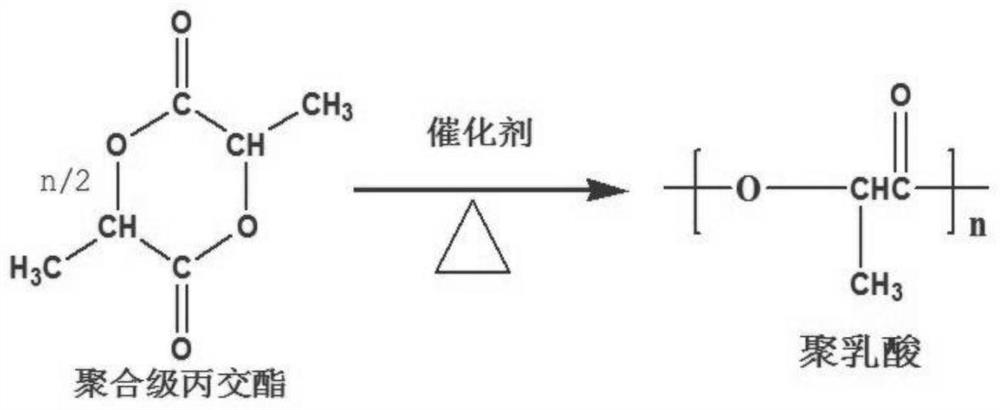
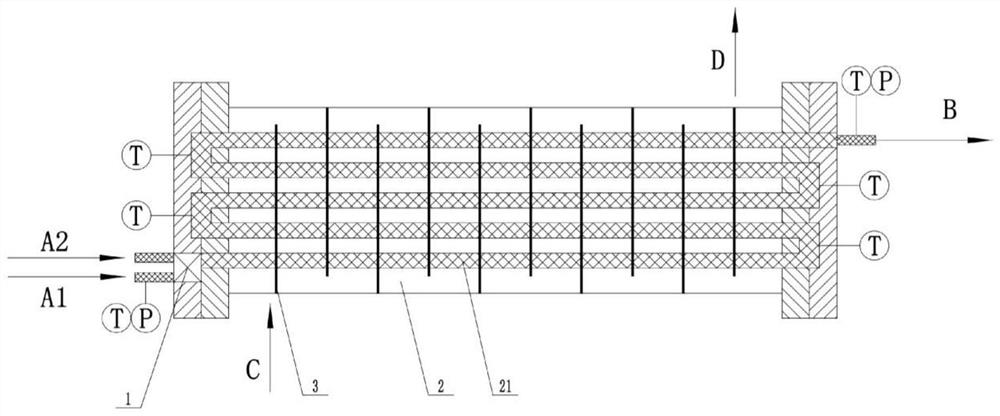
Method for synthesizing polylactic acid by adopting large-channel continuous flow microreactor
A micro-reactor and large-channel technology, applied in the field of organic synthesis, can solve the problems of many side reactions, large molecular weight distribution index, and low production efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
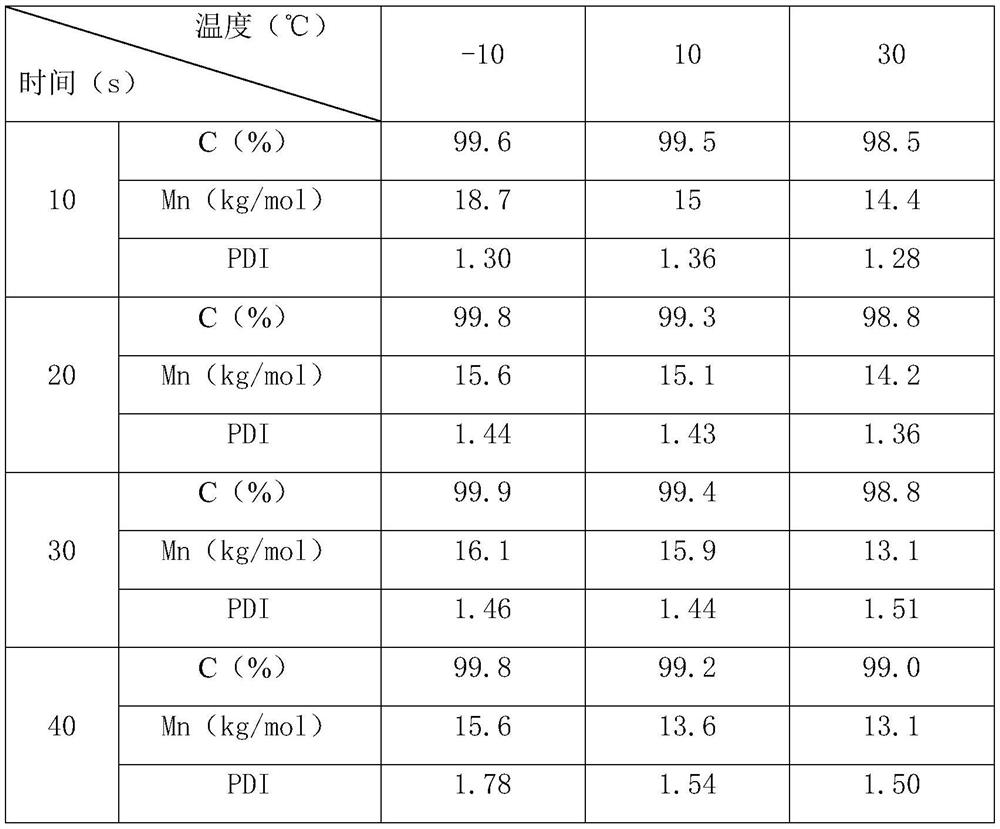
[0037] The loading capacity of catalyst is 1.2mol% of monomer, at reaction temperature-10 ℃, 10 ℃, 30 ℃, residence time 10s, 20s, 30s, 40s, measure its conversion rate C (%), number average molecular weight Mn ( kg / mol) and molecular weight distribution index PDI. The specific data are shown in Table 1:
[0038] Table 1
[0039]
Embodiment 2
[0041] The loading of the catalyst is 0.5mol% of the monomer, at the reaction temperature -10°C, 10°C, 30°C, the residence time is 10s, 20s, 30s, 40s, respectively measure the conversion rate C (%), the number average molecular weight Mn (kg / mol) and molecular weight distribution index PDI. The specific data are shown in Table 2:
[0042] Table 2
[0043]
Embodiment 3
[0045]The load of the catalyst is 0.25mol% of the monomer, at the reaction temperature -10°C, 10°C, 30°C, the residence time is 10s, 20s, 30s, 40s, respectively measure the conversion rate C (%), the number average molecular weight Mn (kg / mol) and molecular weight distribution index PDI. The specific data are shown in Table 3:
[0046] table 3
[0047]
[0048]
[0049] Can find out from embodiment 1-3:
[0050] 1. Under higher catalyst loading (monomer 0.5 mol% and above), the conversion rate of monomer is close to quantitative under all test conditions. When using 1.2mol% catalyst loading, the monomer conversion rate can reach ≥98.5% in the shortest 10s residence time. Residence times in excess of 10 seconds only lead to broadening of the molecular weight distribution.
[0051] 2. At a higher catalyst loading of 1.2mol%, PDI tends to increase, and the optimal reaction parameters can be quickly screened out by using a microreactor. In Example 1, a residence time o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com