Preparation method of chlorpheniramine maleate intermediate
A technology of chlorpheniramine acid intermediate and chlorophenyl, which is applied in the field of preparation of chlorpheniramine maleate intermediate, can solve the problems of multiple by-products, increase the difficulty of purification, affect the yield, etc., and meet the equipment requirements Low, easy to purify, high product purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
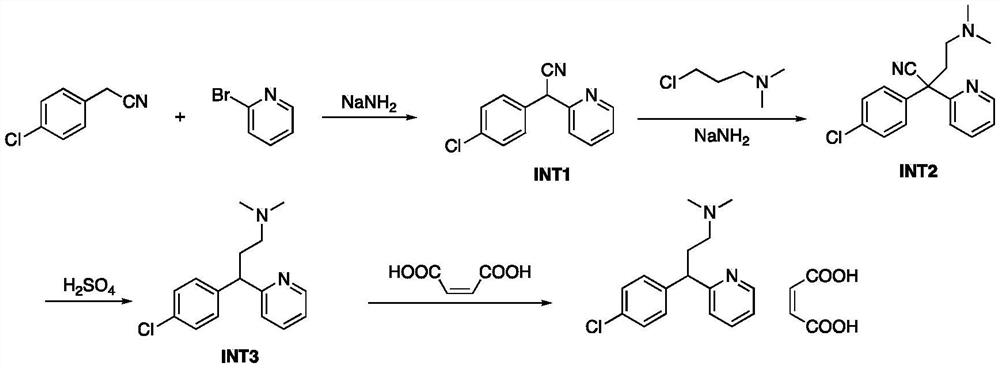
[0043] This embodiment relates to a preparation method of 2-(4-chlorophenyl)-2-(pyridin-2-yl)acetonitrile, comprising the following steps:
[0044] 1) Add p-chlorophenylacetonitrile (90.95g, 1.0eq) into a 1L three-necked flask, add toluene (455mL, 5V), and then NaNH 2 (51.49g, 2.2eq) was added to the three-necked flask, and the reaction was stirred at 25-30°C for 30 minutes, then 2-bromopyridine (104.28g, 1.1eq) was added dropwise to the reaction, and the temperature was controlled below 25-30°C , After the dropwise addition, react at room temperature (25-30° C.), and monitor the reaction by TLC. After the reaction was completed, the reaction system was cooled to room temperature, quenched by adding water (180mL, 2V) and stirred for 30 minutes;
[0045] 2) Add ethyl acetate (270mL*2, 3V*2), separate the liquid, and concentrate the organic phase;
[0046] 3) adding ethyl acetate (15V) to the organic phase;
[0047] 4) Stir in an ice-water bath, add hydrogen chloride ethyl ac...
Embodiment 2
[0050] This embodiment relates to a preparation method of 2-(4-chlorophenyl)-2-(pyridin-2-yl)acetonitrile, comprising the following steps:
[0051] 1) Add p-chlorophenylacetonitrile (90.95g, 1.0eq) into a 1L three-necked flask, add toluene (455mL, 5V), and then NaNH 2 (51.49g, 2.2eq) was added to the three-necked flask, and the reaction was stirred at 25-30°C for 30 minutes, then 2-bromopyridine (104.28g, 1.1eq) was added dropwise to the reaction, and the temperature was controlled below 30-40°C , After the dropwise addition, react at room temperature (25-30° C.), and monitor the reaction by TLC. After the reaction was completed, the reaction system was cooled to room temperature, quenched by adding water (180mL, 2V) and stirred for 30 minutes;
[0052] 2) Add ethyl acetate (270mL*2, 3V*2), separate the liquid, and concentrate the organic phase;
[0053] 3) adding ethyl acetate (15V) to the organic phase;
[0054] 4) Stir in an ice-water bath, add hydrogen chloride ethyl ac...
Embodiment 3
[0057] This embodiment relates to a preparation method of 2-(4-chlorophenyl)-2-(pyridin-2-yl)acetonitrile, comprising the following steps:
[0058] 1) Add p-chlorophenylacetonitrile (90.95g, 1.0eq) into a 1L three-necked flask, add toluene (455mL, 5V), and then NaNH 2 (51.49g, 2.2eq) was added to the three-necked flask, and the reaction was stirred at 25-30°C for 30 minutes, then 2-bromopyridine (104.28g, 1.1eq) was added dropwise to the reaction, and the temperature was controlled below 50-60°C , After the dropwise addition, react at room temperature (85-90° C.), and monitor the reaction by TLC. After the reaction was completed, the reaction system was cooled to room temperature, quenched by adding water (180mL, 2V) and stirred for 30 minutes;
[0059] 2) Add ethyl acetate (270mL*2, 3V*2), separate the liquid, and concentrate the organic phase;
[0060] 3) adding ethyl acetate (15V) to the organic phase;
[0061] 4) Stir in an ice-water bath, add hydrogen chloride ethyl ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com