Organic monomer 1, 3, 6, 8-tetrapyridylpyrene and synthetic method thereof
A synthetic method, the technology of pyridine pyrene, applied in the direction of organic chemistry, etc., can solve the problems of low product solubility, difficulty in product separation and structure analysis, and many by-products, and achieve simple synthesis method, good light absorption capacity, and easy separation and the effect of representation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]Synthesis of 1,3,6,8-tetrapyridine: 30 mL of dioxane and 10 ml of water in three bottles, and then deprive with argon bubbles, 4-pyridine boric acid, 1, 3,6,8-tetraromomatoids and potassium carbonate are added to the mixed solvent in 6 mmol: 1 mmol: 4 mmol of material, and the catalyst thloride and four (triphenylphosphine) palladium are 0.07: 0.15 equivalent ratio. In the above suspension, the mixture was stirred at 90 ° C and stirred under an argon atmosphere, and the reaction was 4 days. The resulting yellow powder crude product was washed with a large amount of methanol solution and filtered, dried at 100 ° C for 6 hours.
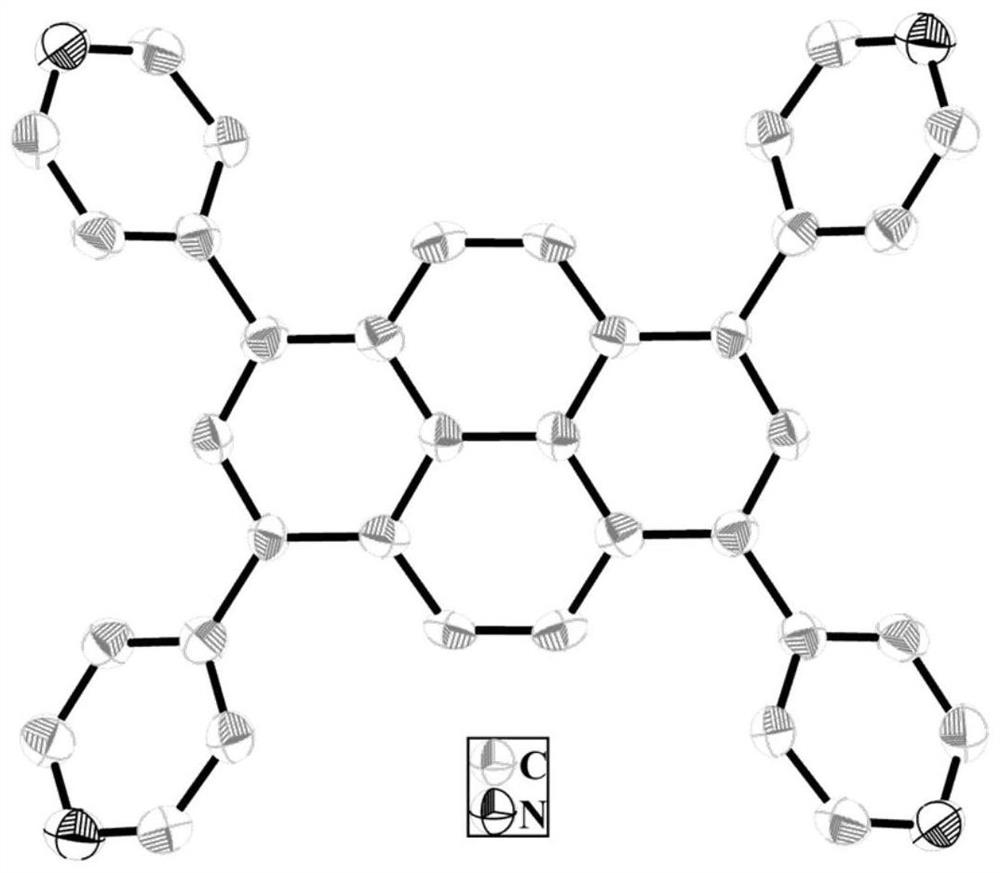
[0022]The dry yellow powder and N, N-dimethylformamide were mixed in a hydrothermal reaction kettle in a hydrothermal reaction kettle, and the temperature was 100 ° C, the temperature was 15 hours, fell to room temperature. Filter, the resulting large amount of yellow crystal is the product 1,3,6,8-tetrapyridine 芘 (figure 1 ).
[0023]The synthetic routes of ...
Embodiment 2
[0026]Synthesis of 1,3,6,8-tetrapyridine: 30 mL of dioxane and 10 ml of water in three bottles, and then deprive with argon bubbles, 4-pyridine boric acid, 1, 3,6,8-tetraromomatoids and potassium carbonate were added to the mixed solvent in 5 mmol: 1 mmol: 4 mmol, and the catalyst tetra (triphenylphosphine) palladium was added to the above-mentioned suspension in an equivalent of 0.18. The mixture was refluxed under 93 ° C under the argon atmosphere, and the reaction was reacted for 3.5 days. The resulting yellow powder crude product was washed with a large amount of methanol solution and filtered, dried at 100 ° C for 6 hours.
[0027]The dry yellow powder crude product and N, N-dimethylformamide were mixed in a hydrothermal reaction kettle in a hydrothermal reaction kettle, and the temperature was 90 ° C, the heating reaction time was 12 hours, down to room temperature. Filtration, a large amount of yellow crystals, i.e., product 1,3,6,8-tetrapyridine.
Embodiment 3
[0029]Synthesis of 1,3,6,8-tetrapyridine: 30 mL of dioxane and 10 ml of water in three bottles, and then deprive with argon bubbles, 4-pyridine boric acid, 1, 3,6,8-tetraromomatoids and potassium carbonate were added to the mixed solvent in 4 mmol: 1 mmol: 4 mmol of material, and the catalyst tetra (triphenylphosphine) palladium was added to the above-mentioned suspension in an equivalent of 0.15. The mixture was stirred at 90 ° C under the argon atmosphere, and the reaction was 4 days. The resulting yellow powder crude product was washed with a large amount of methanol solution and filtered, dried at 100 ° C for 6 hours.
[0030]The rough product and N, N-dimethylformamide were mixed in a hydrothermal reaction kettage, and the temperature was 100 ° C, the heating reaction time was 24 hours, and the heated reaction time was filtered after 5 mg. The resulting large amount of yellow crystals, i.e., product 1,3,6,8-tetrapyridine.
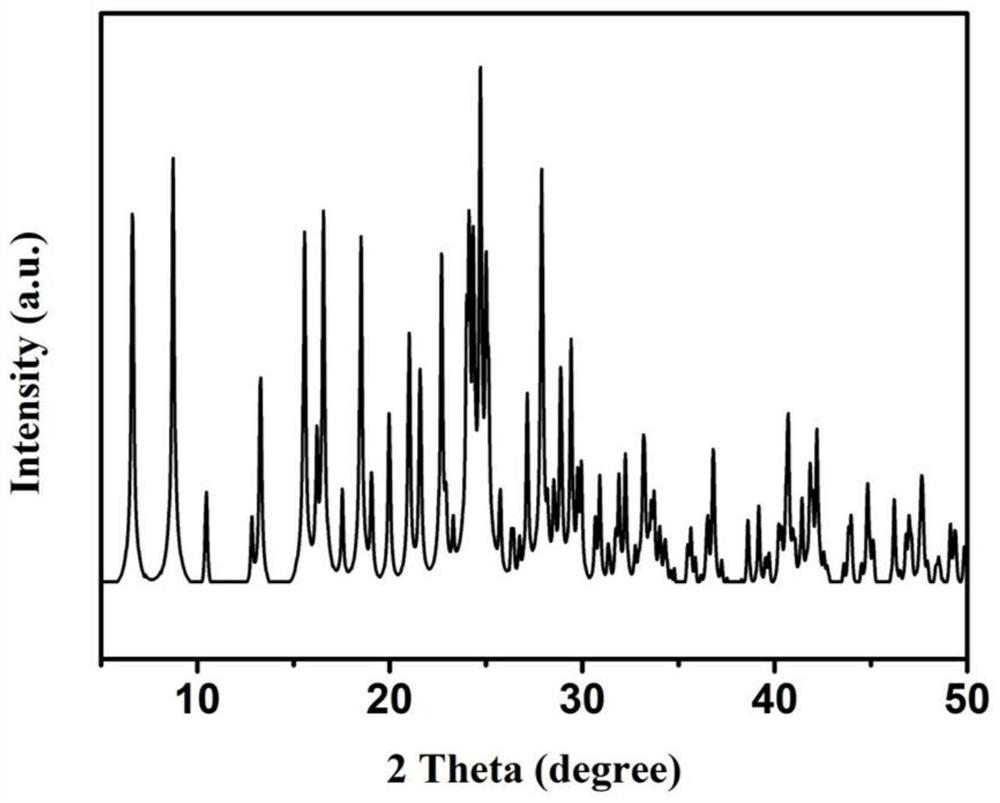
[0031]Structural assay and characterization of three, 3, 6, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com