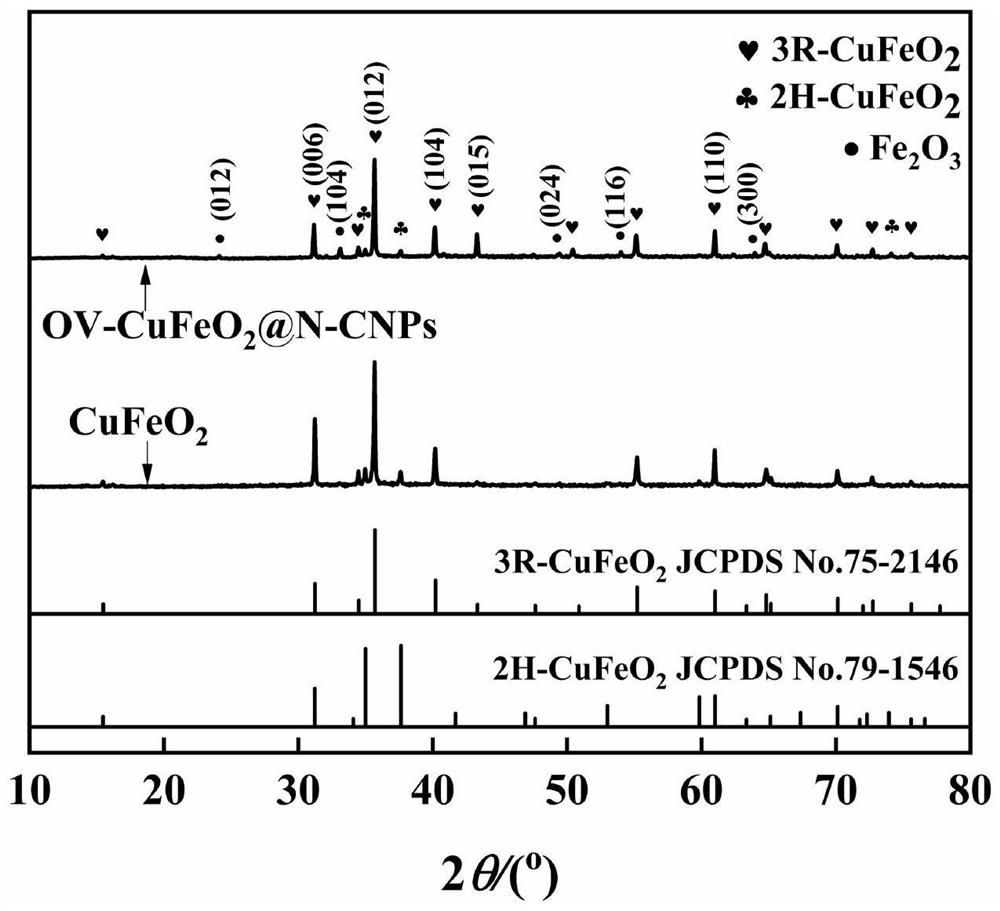
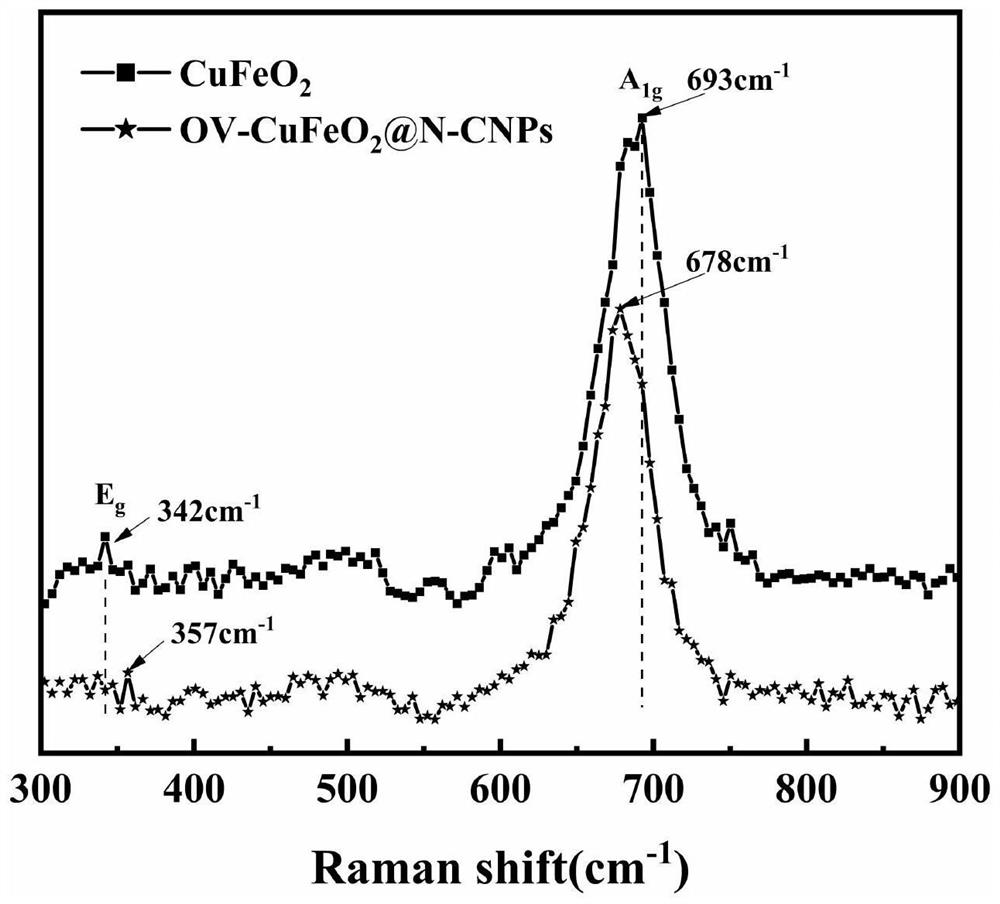
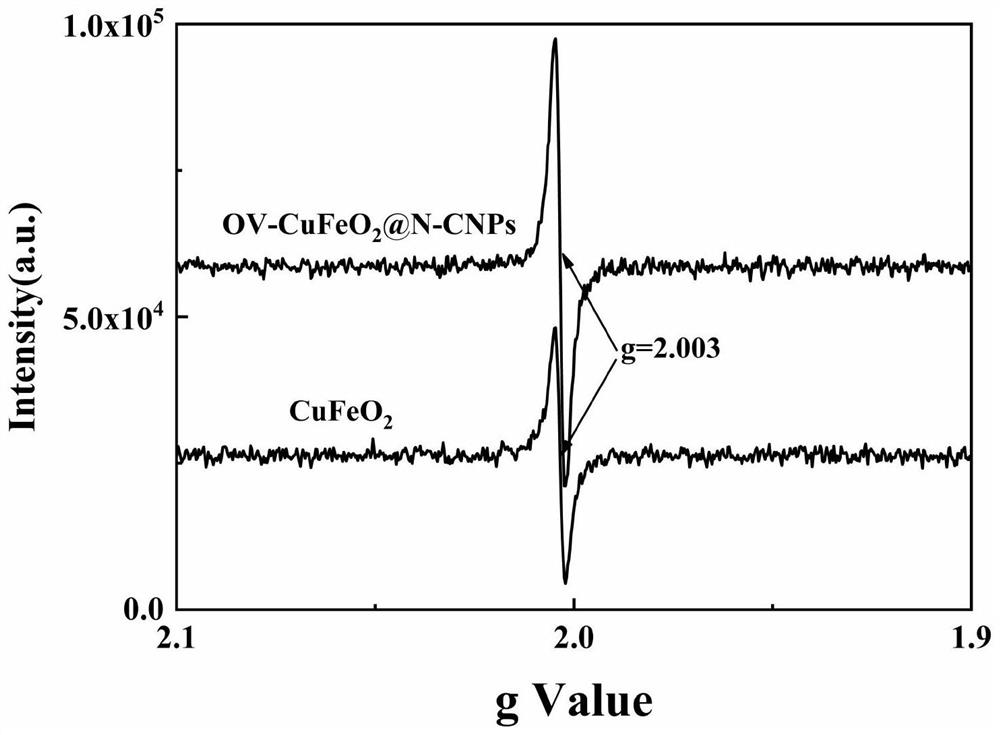
Modified cuprous ferrite catalystrich in oxygen vacancies as well as preparation method and application thereof
A cuprous ferrite, catalyst technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc. The effect of reducing the dosage and improving the degradation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The invention provides a kind of preparation method of modified cuprous ferrite catalyst, comprising the following steps:
[0029] mixing the chitosan solution and the iron-copper metal salt solution, dropping the resulting mixed solution into alkali, and carrying out a precipitation reaction to obtain an iron-copper precipitation mixed solution coated with chitosan;
[0030] The chitosan-coated iron-copper precipitation mixed solution is subjected to hydrothermal reaction and dried to obtain a modified cuprous ferrite catalyst.
[0031] In the present invention, unless otherwise specified, the required preparation materials are commercially available products well known to those skilled in the art.
[0032] The invention mixes the chitosan solution and the iron-copper metal salt solution, drops the obtained mixed solution into the alkali, carries out the precipitation reaction, and obtains the iron-copper precipitation mixed solution coated with the chitosan. In the p...
Embodiment 1
[0045] 200mg chitosan was dissolved in 20mL 2% glacial acetic acid solution, and left to stand overnight to obtain chitosan solution; weigh 5mmol Cu(NO 3 ) 2 ·3H 2 O and 5 mmol FeSO 4 ·7H 2 O was dissolved in 10mL ultrapure water, and the obtained iron-copper metal salt solution was slowly added to the chitosan solution, stirred to make it evenly mixed, and then the obtained mixed solution was dropped into 30mL1.67mol / L NaOH aqueous solution drop by drop. , carry out the precipitation reaction at room temperature for 60 minutes, the obtained iron-copper precipitation mixed solution coated with chitosan is sonicated for 10 minutes, and then put into a stainless steel reaction kettle with a polytetrafluoroethylene liner, and carry out hydrothermal reaction at 180 ° C for 12 hours. The obtained product was centrifugally washed until the surface of the product was neutral, and dried in vacuum at 60°C for 12 hours to obtain a modified cuprous ferrite catalyst, which was denoted ...
Embodiment 2
[0047] The difference between this embodiment and Example 1 is only that the amount of chitosan added is 100 mg, and the prepared catalyst is denoted as OV-CuFeO 2 @N-CNPs-100; In the prepared modified cuprous ferrite catalyst, the loading amount of nitrogen-doped carbon nanoparticles on the cuprous ferrite is 9.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com