Quinoline derivative, preparation method and application thereof in prevention and treatment of plant viruses and sterilization
A kind of derivative, quinoline technology, applied in the field of pesticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
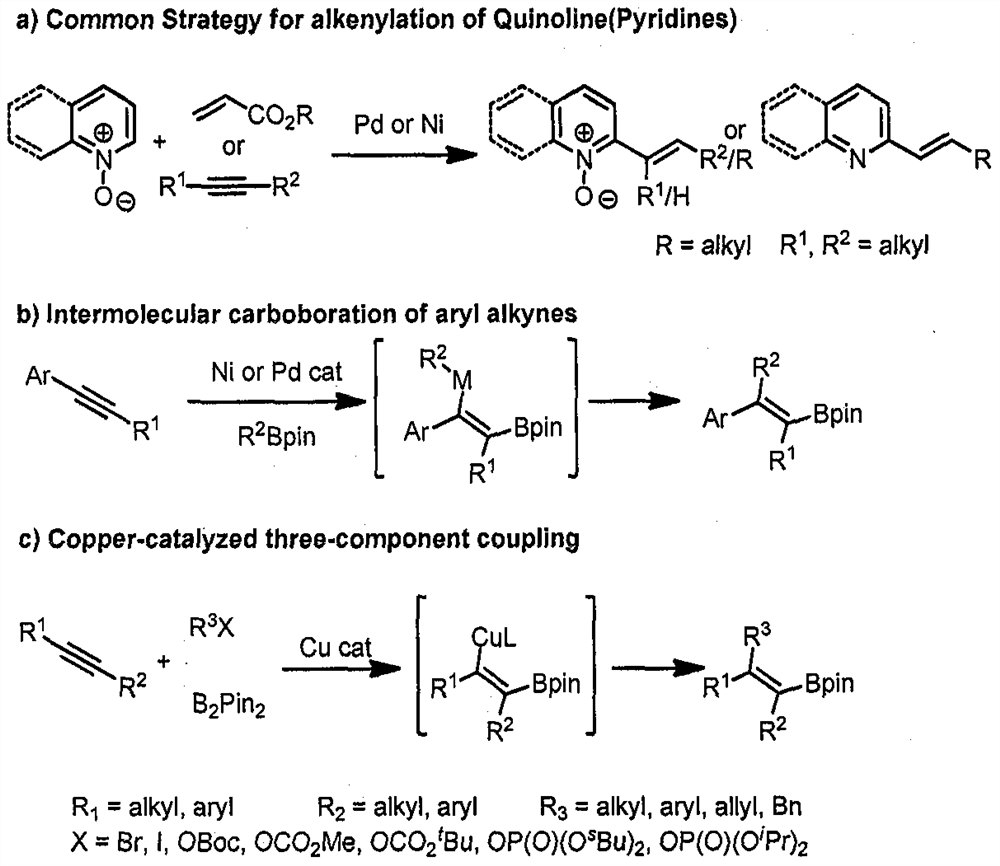
[0040]The present invention provides a method of preparing a 2-position alkenyl substituted quinoline derivative, including: in the presence of a catalyst, boron reagent, ligand, base, in an organic solvent, a quine (II) The arylioxide represented by the nitride formula to the formula (III) to obtain a compound represented by the formula (I) by selective hydrocarbon bonding.
[0041]
[0042]According to the invention, the compound represented by the formula (II) and the formula (III) can be specifically selected according to the desired formula (I), and R1R2R3R4R5R6And R as described above, the present invention will not be described later.
[0043]Among them, the molar ratio of the quinoline nitrogen oxide shown in the formula (II) and the dirylene of formula (III) is from 1 to 3: 1, preferably from 1 to 2: 1;
[0044]Preferably, the catalyst is Cucl, Cubr, Cui, Cu (OAC)2Cu (OTF)2, Cu (Acac)2One or more of IMES-CUCL, IPR-CUCL;
[0045]The amount of the catalyst can fluctuate in a wider range, su...
Embodiment 1
[0079](E) -2- (1,2-dimstyrene) quinoline (IAA) synthesis
[0080]Quinoline nitrogen oxide IIA (1.5 eq), alkyne (10 mol%), Cucl (10 mol%), Cucl (10 mol%), Cucl (10 mol%), Cucl (10 mol%)3(12 mol%), B2Pin2(1.5 eq), Buok (2.0 eq), 2-methf (0.2 M), after the air was replaced with AR 3 times, heated to 80 ° C, and the reaction was 24 h. After the reaction is complete, the solvent is evaporated and chromatography directly. White solid, melting point 114-115 ° C, 126.0 mg of mass, and 82% yield.1H NMR (400MHz, CDCL3Δ8.17 (D, J = 8.4 Hz, 1H), 7.98 (D, J = 8.8 Hz, 1H), 7.96 (S, 1H), 7.75 (D, J = 8.0 Hz, 1H), 7.74-7.69 ( M, 1H), 7.52-7.47 (m, 1H), 7.44-7.37 (m, 3H), 7.34-7.28 (m, 2H), 7.17-7.12 (m, 5H), 7.10 (D, J = 8.8 Hz, 1h);13C NMR (100MHz, CDCL3Δ159.3, 148.1, 141.1, 139.4, 137.0, 136.1, 132.6, 130.8, 130.3, 129.8, 127.8, 127.6, 127.5, 127.4, 126.3, 121.0.HRMS (ESI): Calcdfor C23Hide18N [m + h]+: 308.1434; Found: 308.1439.
[0081](E) -2- (1,2-double (4-fluorophenyl) vinyl) quinoline (IAB)
[0082]...
Embodiment 2
[0126]Example 2: Determination of anti-tobacco leaf virus activity, as follows:
[0127]1. Virus purification and concentration measurement:
[0128]Refer to the Tobacco Flower Leaf Virus SOP Specification Computed by Nankai University Elements. After the virus crude liquid was centrifuged by 2 polyethylene glycol, the concentration was measured, then reflected at 4 ° C.
[0129]2. Compound solution formulated:
[0130]After weighing, the primary drug was dissolved with DMF with 1 × 105 μg / ml of mother liquor, which was then diluted with 1 ‰ Tween 80 aqueous solution to the desired concentration; Ningnamycin formulation Dilution directly against water.
[0131]3. Friction vaccination of Shanxi smoked blade, rinse with water, virus concentration 10 μg / ml. After cutting, cut, along the leaf medal, and the left and solenoids were immersed in 1 ‰ Temperature water and the medicament. After 30 minutes, the moisturizing culture was moisturized at a suitable illumination temperature, each three leave...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com