NTR-1 response type fluorescent probe based on benzoindole as well as preparation method and application thereof
A technology of benzindole and fluorescent probes, which is applied in the field of preparation and fluorescent probes, can solve the problems of low sensitivity, low fluorescence quantum yield, and poor stability, and achieve high-sensitivity detection, significant fluorescence enhancement, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthesis of embodiment 1 probe NFP-1:
[0037] 1. Synthesis of 1-ethyl-2-methylbenzo[cd]indole-1-chloride (EMC)
[0038] 1,8-Naphtholactam (8.03g, 47.4mmol) was dissolved in 100mL DMF, and sodium hydride (3.528g, 150mmol) was added slowly at 0°C. After the system was warmed up to room temperature, ethyl iodide (7.403 g, 50 mmol) was added dropwise and stirred at room temperature for 1 h. The reaction mixture was extracted with ethyl acetate (150mL×2), the organic layer was washed with saturated brine and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure, and the obtained solid was subjected to silica gel column chromatography (petroleum ether: ethyl acetate=10 : 1) Separation and purification to obtain 1-ethylbenzo[cd]indol-2(1H)-one 3 (7.762g yellow solid, yield 83%), which was directly used in the next reaction.
[0039] 3 (1.972 g, 10 mmol) was dissolved in 40 mL of anhydrous THF (40 mL), and methylmagn...
Embodiment 2
[0044] Spectral properties of embodiment 2 probe NFP-1:
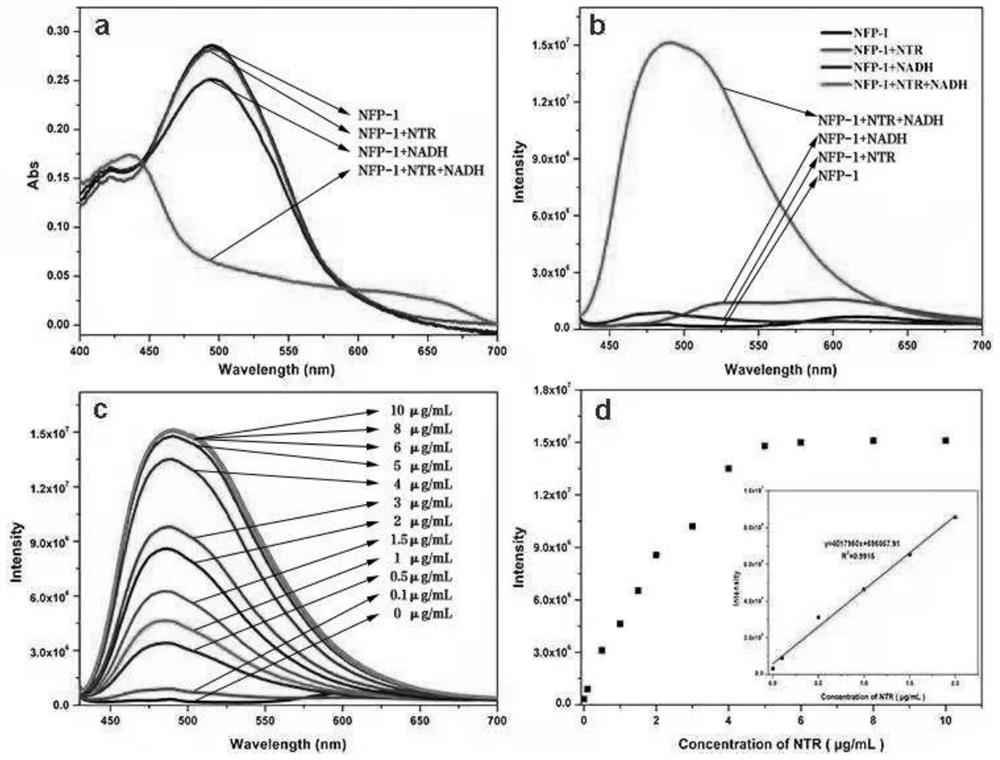
[0045] All UV-Vis absorption and fluorescence spectra of probe NFP-1 were recorded in solution DMSO-PBS (1:9 v / v, 10 mM, pH=7.4). at lambda ex =420nm collects the fluorescence emission of the solution at 430nm-700nm, the excitation and emission slit width is 5nm / 5nm. NFP-1 was prepared as 1 mM stock solution in DMSO and then diluted for use. NTR was dissolved in ultrapure water to prepare a 100 μg / mL stock solution for later use. Unless otherwise specified, 500 μM NADH was added to all reactions.
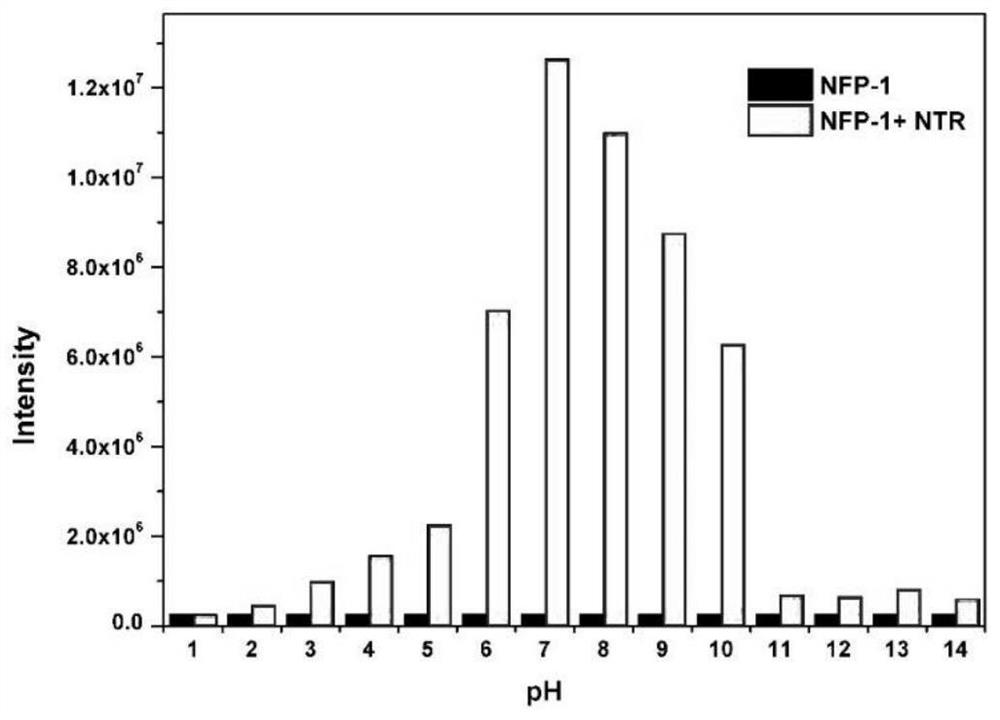
[0046] NADH (500 μM) was added to NFP-1 (10 μM), and then incubated with different concentrations of NTR (0-10 μg / mL) at 37°C for 30 min, and the UV-visible absorption spectrum and fluorescence spectrum were recorded. In the selectivity study, NFP-1 (10 μM) was incubated with NTR (5 μg / mL), reactive oxygen species (500 μM), some metal ions (1 mM), amino acids (1 mM) at 37 ° C for 30 min, and then recorded their fluorescen...
Embodiment 3
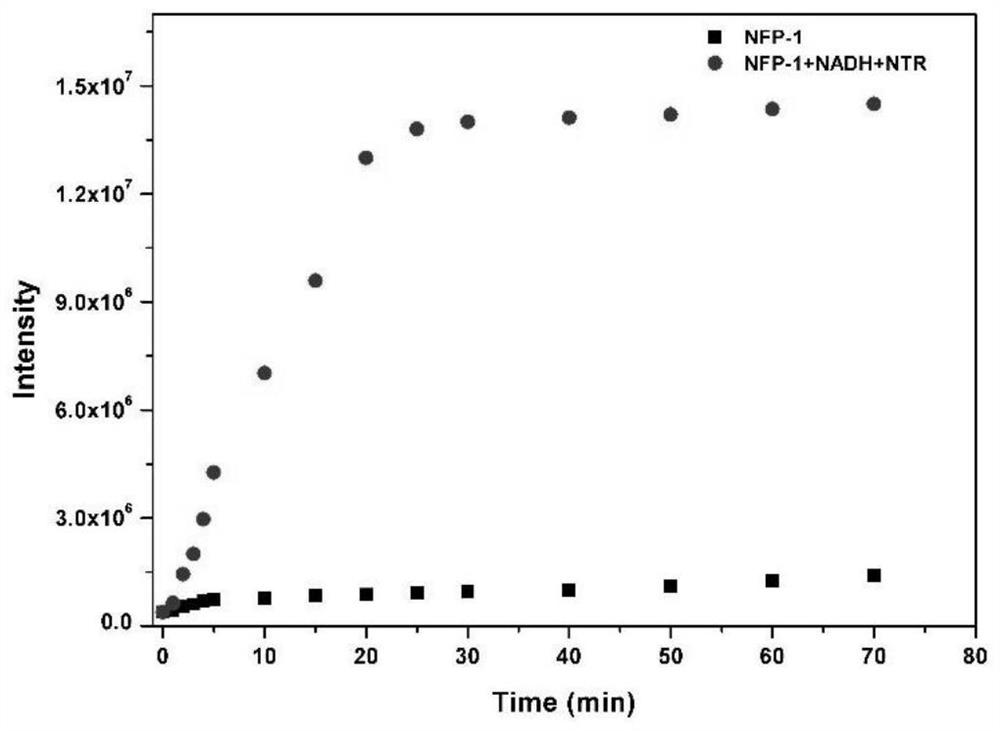
[0052] The kinetic response of embodiment 3 probe NFP-1:
[0053] In order to study the kinetic response of probe NFP-1 to NTR, the fluorescence intensity I 490nm The change. Such as figure 2shown. The results showed that the stability of the probe NFP-1 was better, and the I of the probe after adding NTR 490nm It increased with increasing response time and reached a plateau at 25 minutes. The above analysis indicated that the probe NFP-1 could completely react with NTR within 25 min. To ensure that the reaction proceeded completely, a reaction time of 30 min was chosen in all response experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com